0392
Age-related neuropathologies associated with white matter hyperintensities burden: a study of a community cohort of older adults.1Department of Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, United States, 2Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, IL, United States, 3Department of Neurological Sciences, Rush University Medical Center, Chicago, IL, United States, 4Department of Pathology, Rush University Medical Center, Chicago, IL, United States, 5Department of Diagnostic Radiology, Rush University Medical Center, Chicago, IL, United States
Synopsis
White matter hyperintensities (WMH) are lesions commonly observed in the brain of older adults, and have been associated with lower cognitive function, lower motor performance, and increased risk of dementia. The purpose of this work was to investigate the neuropathologic correlates of WMH burden by combining ex-vivo MRI and pathology on a large community cohort of older adults.
Purpose
White-matter hyperintensities (WMH) are commonly observed in the brain of older adults and have been associated with lower cognitive function, lower motor performance, and increased risk of dementia1-4. A number of studies have combined brain MRI with measures of neuropathology to assess the neuropathologic correlates of WMH5-7. However, these studies were characterized by one or more of the following limitations: a) use of clinical cohorts (results may not be representative of the general population), b) low numbers of participants (low statistical power), c) focus on one or few pathologies (results may be biased towards these pathologies), d) long intervals between in-vivo MRI and autopsy (additional pathology may have developed), e) frail individuals tend to be excluded from in-vivo MRI research (results may be biased). Therefore, the purpose of this work was to examine the association between WMH and age-related neuropathologies using a study design that addresses the above shortcomings by combining ex-vivo MRI and pathology in a large community cohort of older adults.Methods
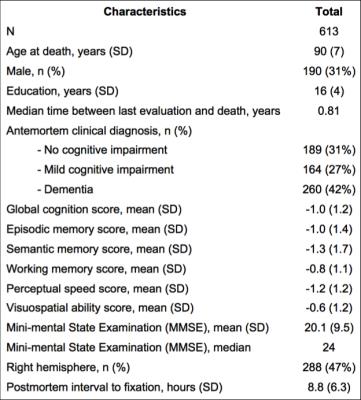
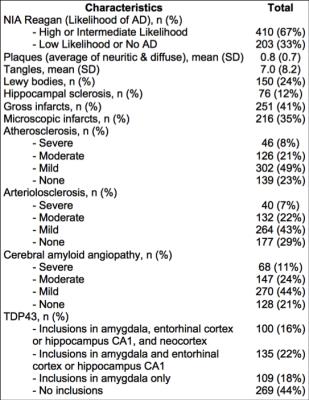
Cerebral hemispheres were obtained from 613 deceased participants of two longitudinal, epidemiologic, clinical-pathologic cohort studies of aging (Fig.1). All hemispheres were imaged ex-vivo on a 3T MRI scanner, while immersed in 4% formaldehyde solution. Following imaging, all hemispheres underwent neuropathologic examination (Fig.2). One rater was trained by an expert to rate WMH in periventricular and deep white matter separately, based on ex-vivo MR images, according to the original Fazekas scale. The overall WMH burden was defined as the maximum of the periventricular and deep white matter ratings, and was used in analyses. Intraclass correlation was used to assess the intra-rater reliability and agreement with the expert. Ordinal logistic regression was used in the whole group (N=613) to test the association of the overall WMH burden with amyloid plaques, PHF-tau tangles, Lewy bodies, TDP43, hippocampal sclerosis, gross infarcts, microscopic infarcts, atherosclerosis, arteriolar sclerosis, and cerebral amyloid angiopathy, controlling for age at death, sex, education, and postmortem interval to fixation. The same analysis was repeated in the non-demented participants (N=353), as well as in participants with no cognitive impairment (N=189), separately.Results
Intra-rater agreement was strong (ICC=0.75), and agreement with the expert was moderate to high (ICC=0.64). When considering the whole group, overall WMH burden was significantly associated with amyloid plaques (0.34, p=0.03), gross infarcts (0.50, p<10-4), microscopic infarcts (0.24, p=0.04), and arteriolar sclerosis (0.50, p<10-4). When considering only non-demented participants, overall WMH burden was significantly associated with gross infarcts (0.67, p<10-4), microscopic infarcts (0.31, p=0.05), and arteriolar sclerosis (0.64, p<10-4). Finally, when considering only participants with no cognitive impairment, overall WMH burden was significantly associated with gross infarcts (0.82, p=0.0008) and arteriolar sclerosis (0.49, p=0.01).Discussion
When considering both demented and non-demented participants, overall WMH burden was associated with both neurodegenerative (amyloid plaques) and vascular pathologies (infarcts and arteriolar sclerosis). However, in persons without dementia overall WMH burden was associated only with vascular pathologies (infarcts and arteriolar sclerosis). This suggests that, in non-demented persons, presence of WMH in MRI may be a marker of vascular pathologies (infarcts and arteriolar sclerosis). Furthermore, the finding in persons with no cognitive impairment that WMH burden was associated with gross infarcts and arteriolar sclerosis, suggests that an older adult with no cognitive impairment having an MRI scan with WMH but no gross infarcts, may have high likelihood to be suffering by arteriolar sclerosis.
The present study provides robust evidence on the neuropathologic correlates of WMH in a community cohort of older adults. Combination of ex-vivo MRI and pathology allowed us to a) include a large number of persons from community cohorts, b) include a large number of non-demented individuals, c) include older adults independent of frailty level, d) consider multiple pathologies, e) eliminate the interval between imaging and autopsy. To our knowledge, this is the largest MRI-pathology investigation to date.
Acknowledgements
National Institute of Neurological Disorders and Stroke (NINDS) UH2NS100599
National Institute on Aging (NIA) R01AG017917
National Institute on Aging (NIA) P30AG010161
National Institute on Aging (NIA) R01AG034374
References
1. The LADIS study group, Poggesi A, Pantoni L, et al. 2001-2011: A Decade of the LADIS (Leukoaraiosis And DISability) Study: What Have We Learned about White Matter Changes and Small-Vessel Disease? Cerebrovasc Dis 2011;32:577-588.
2. Silbert LC, Nelson C, Howieson DB, et al. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology 2008;71:108-113.
3. Arvanitakis Z, Fleischman DA, Arfanakis K, et al. Association of white matter hyperintensities and gray matter volume with cognition in older individuals without cognitive impairment. Brain Struct Funct 2016;221:2135-2146.
4. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010;341:c3666.
5. Erten-Lyons D, Dodge HH, Woltjer R, et al. Neuropathologic basis of age-associated brain atrophy. Neurology 2013;70:616-622.
6. Shim YS, Yang DW, Roe CM, et al. Pathological correlates of white matter hyperintensities on magnetic resonance imaging. Dement Geriatr Cogn Disord 2015;39:92-104.
7. Polvikoski TM, van Straaten EC, Barkhof F, et al. Frontal lobe white matter hyperintensities and neurofibrillary pathology in the oldest old. Neurology 2010;75:2071-2078.