0382
Extended RF shimming: Sequence level parallel transmission optimization applied to steady state free precession MRI of the heart1Biomedical Engineering and imaging Sciences, King's College London, London, United Kingdom, 2Centre for the Developing Brain, King's College London, London, United Kingdom
Synopsis
Balanced steady-state free precession (bSSFP) cardiac MRI benefits greatly from reduced repetition time (TR). Minimum TR is often limited by specific absorption rate (SAR) and hardware constraints. RF shimming can be used with parallel transmission (PTx) to work within such constraints, but direct minimization of TR is not straightforward since the constraints themselves vary as TR is reduced.
We present an extended RF shimming framework in which PTx degrees of freedom are simultaneously optimised with pulse sequence properties. The result is minimum TR bSSFP sequences that operate at the SAR limits and within hardware constraints for 3T cardiac MRI.
Purpose
Reduction of repetition time (TR) for balanced-steady-state-free-precession (bSSFP) cardiac MRI sequences can be directly translated to reduced breath-hold durations and fewer banding artefacts1. TR for bSSFP is usually limited by specific absorption rate (SAR) and hardware constraints; including peak and average power and RF duty cycle2. Parallel transmission (PTx) has been demonstrated for optimization of RF pulses within such constraints3, however reduction of sequence TR is not straightforward because the constraints themselves vary with sequence properties. We propose a ‘sequence level’ approach to RF shimming for cardiac bSSFP. The approach jointly estimates optimal RF shim settings and sequence properties (pulse duration; $$$\tau$$$) such that overall TR is minimized.Theory
For a PTx system with $$$N_c$$$ channels, RF shimming is the process of determining an optimal set of complex dimensionless weighting $$$\mathbf{w}_j (j=1:N_c)$$$ to apply to each channel.
For a sequence with a given TR and RF pulse shape $$$p(t)$$$ we may write the constraints on $$$\mathbf{w}$$$ as a function of pulse duration ($$$\tau$$$):
\[constraints_{\mathbf{w}}(\tau):\left\{\begin{array}{ll}\max\limits_{i}\left\lbrace\mathbf{w^*Q_iw}\right\rbrace\times LF^2\times\dfrac{\theta_0^2}{\gamma^2}\times\dfrac{\delta_2}{\delta_1^2} \times\dfrac{1}{\tau TR}\leq lSAR_{max}\\\left\lbrace\mathbf{w^*Q_{wb}w}\right\rbrace\times LF^2\times\dfrac{\theta_0^2}{\gamma^2}\times\dfrac{\delta_2}{\delta_1^2} \times\dfrac{1}{\tau TR}\leq wbSAR_{max}\\|\mathbf{w}_j|\leq\tau\dfrac{P_{peak}}{\sqrt{A}} \dfrac{\delta_1\gamma}{\theta_0}\quad\forall j\\|\mathbf{w}_j|\leq\sqrt{\tau TR}\dfrac{\delta_1\gamma}{\theta_0\sqrt{\delta_2}}\sqrt{\dfrac{P_{avg}}{A}}\quad\forall j\end{array}\right.\]
Top-to-bottom these expressions represent local SAR (10g averaged), whole-body SAR, peak power and average power respectively.
$$$\mathbf{Q_{wb}}$$$ and $$$\mathbf{Q_{i}}$$$ are the whole body and local SAR matrices4,5 and $$$\theta_0$$$ the target flip angle. $$$\delta_1$$$ and $$$\delta_2$$$ are intrinsic properties of $$$p(t)$$$ relative to a block pulse with the same flip angle and amplitude ($$$\delta_1$$$ is the relative duration, $$$\delta_2$$$ is the relative energy). $$$lSARmax$$$ and $$$wbSARmax$$$ are the local and whole-body SAR limits; $$$P_{peak}$$$ and $$$P_{avg}$$$ are peak and average RF power limits. $$$LF$$$ is a scalar ‘loading factor’ to match the SAR model and $$$A$$$ is a scalar that quantifies RF chain efficiency in W/$$$\mu$$$T2.
The minimum TR is set by:
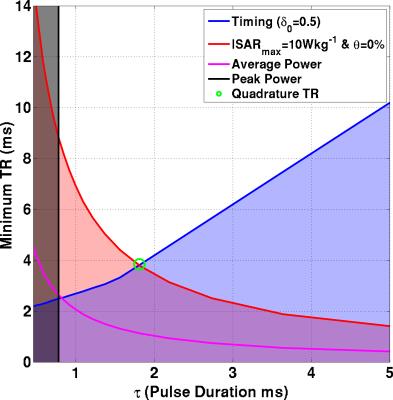
\[TR_{min} = max\left\{\begin{array}{ll}\tau+t_{enc}\\\tau/\delta_0\end{array}\right\}\]
$$$t_{enc}$$$ is time required for image encoding and $$$\delta_0$$$ is the RF amplifier gating duty-cycle limit (50%). Figure 1 illustrates the intersection of these constraints for a single-channel system – the green circle indicates the TR that would be selected for scanning.
The optimization is performed using a nested approach with an outer step that seeks to find the lowest acceptable $$$\tau$$$ to minimize sequence TR:
$$\mathrm{arg\,min}_{\tau}\left\lbrace TR+f(\hat{\theta})\right\rbrace$$
where $$$f(\hat{\theta})$$$ is a function that penalises solutions with high bias $$$(\hat{\theta})$$$ in achieved flip angle within a region of interest (ROI). For each $$$\tau$$$, an inner optimization seeks optimal $$$\mathbf{w}$$$ given the constraints. This was done in two ways – one minimises mean squared error (MSE) in $$$\theta$$$:
$$\begin{align} \mathrm{arg\,min}&_{\mathbf{w}}\||\mathbf{S_{\theta}w}|-\mathbf{\theta_0}\|^2_{ROI}\\ \mathrm{s.t.}&\quad constraints_{\mathbf{w}}(\tau) \end{align}$$where $$$\mathbf{S_{\theta}}$$$ represents spatially dependent RF sensitivities of the transmit channels in units of achieved flip angle. Although MSE improves flip angle homogeneity in the target region, it cannot achieve a zero bias solution (5% bias was allowed). The second optimisation minimizes bias $$$\hat{\theta}$$$ alone:
$$\begin{align}\mathrm{arg\,min}&_{\mathbf{w}}\|\overline{|\mathbf{S_{\theta}w}|}-\theta_0\|^2_{ROI}\\ \mathrm{s.t.}&\quad constraints_{\mathbf{w}}(\tau)\end{align}$$
Methods
Experiments performed on a Philips 3T MRI system with an 8-channel transmit body array6 and 6-channel receiver array. Seven male subjects were scanned; each matched to one of two pre-calculated SAR models based on their body mass index (a smaller model (BMI=23.5) for subjects 1-5 and a larger model (BMI=31) for subjects 6&7).
B$$$_1^+$$$ mapping used DREAM7 and bSSFP imaging with $$$\theta_0$$$=45° was performed for a 4-chamber cardiac view. The ROI was manually drawn to only include myocardium. Optimal sequence parameters for the transmit coil quadrature mode (similar to a whole-body birdcage) were also determined using the outlined approach (Figure 1).
Results
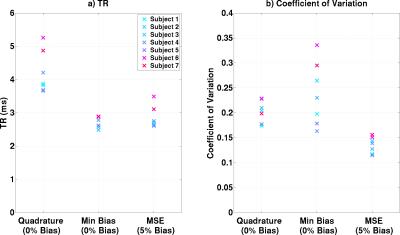
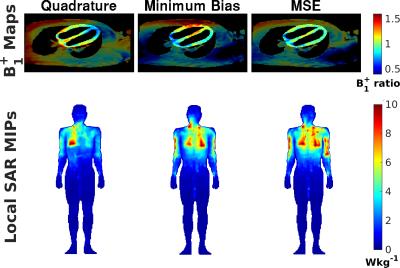
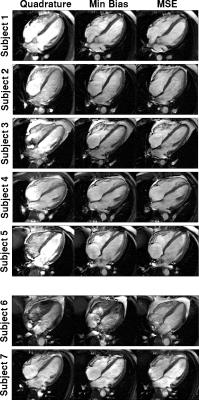
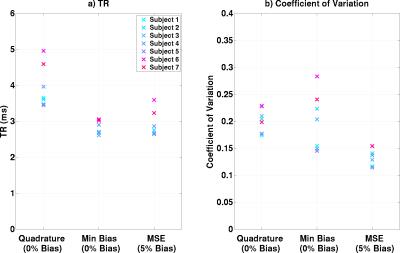
Figure 2a shows minimum TRs achieved in the three scenarios. Both optimisations substantially reduce TR compared with quadrature mode (TR reduced by 1.35±0.31ms) with the minimum bias optimisation performing better. However, minimum bias optimization leads to unpredictable flip-angle uniformity (Figure 2b). Figure 3 shows B$$$_1^+$$$ maps from one subject, illustrating this effect, along with predicted $$$lSARmax$$$ distributions – all limited by 10Wkg-1. Figure 4 shows bSSFP images for all subjects.
The method can be used with different SAR limits – repeating the optimization for $$$\theta_0$$$=60° with $$$lSARmax$$$=20Wkg-1 as in Reference 8 leads to the results in Figure 5. In this case minimum TRs of 2.7ms are possible, comparing favourably with the published 3.8ms for the same constraints.
Discussion and Conclusions
An extended RF shimming framework was proposed that reduces TR in bSSFP scans by finding optimal pulse sequence properties in conjunction with RF shimming. Figure 3 shows that while remaining within the maximum local SAR limits, the optimized sequences operate closer to the whole-body SAR limit, effectively making the sequence more efficient. The method is applicable in principle to any gradient-echo sequence, and for a range of different hardware and safety limits.Acknowledgements
The research was funded by the following grant:
EPSRC (UK research councils) grant number EP/L00531X/1
References
1. Bieri O, Scheffler K. Fundamentals of balanced steady state free precession. J. Magn. Reson. Imaging. 2013;38:2–11.
2. Schär M, Kozerke S, Fischer SE, Boesiger P. Cardiac SSFP Imaging at 3 Tesla. Magn. Reson. Med. 2004;51:799–806.
3. Brunner DO, Pruessmann KP. Optimal design of multiple-channel RF pulses under strict power and SAR constraints. Magn. Reson. Med. 2010;63:1280–91.
4. Graesslin I, Homann H, Biederer S, Börnert P, Nehrke K, Vernickel P, Mens G, Harvey P, Katscher U. A specific absorption rate prediction concept for parallel transmission MR. Magn. Reson. Med. 2012;68:1664–1674.
5. Eichfelder G, Gebhardt M. Local specific absorption rate control for parallel transmission by virtual observation points. Magn. Reson. Med. 2011;66:1468–76.
6. Vernickel P, Röschmann P, Findeklee C, Lüdeke K-M, Leussler C, Overweg J, Katscher U, Grässlin I, Schünemann K. Eight-channel transmit/receive body MRI coil at 3T. Magn. Reson. Med. 2007;58:381–9.
7. Nehrke K, Bornert P. DREAM-a novel approach for robust, ultrafast, multislice B1 mapping. Magn. Reson. Med. 2012;68:1517–1526.
8. Weinberger O, Winter L, Dieringer MA, et al. Local Multi-Channel RF Surface Coil versus Body RF Coil Transmission for Cardiac Magnetic Resonance at 3 Tesla: Which Configuration Is Winning the Game? PLoS One. 2016;11:e0161863.
Figures