0377
Diffusion Tensor Imaging of the Anterior Cruciate Ligament Graft1Dept. of Radiology, University Hospital Antwerp, Edegem, Belgium, 2Dept. of Radiology, University Medical Center Utrecht, Netherlands, 3Dept. of Orthopedics, Monica Orthopedic Research (MoRe) Foundation, Monica Hospital, Belgium, 4Vision Lab, Dept. of Physics, University of Antwerp, Belgium
Synopsis
Anterior cruciate ligament (ACL) reconstruction using a tendon graft remains the standard of care for ACL injuries. Postoperatively, the graft undergoes a biologic transition from tendinous to ligamentous in appearance. Despite substantial research efforts, little is known about the human ACL graft ligamentization process. Much of the current knowledge on graft ligamentization have been derived from biopsy studies. However, biopsies are invasive and suffer from sampling error. Our study demonstrates the feasibility and reliability of diffusion tensor imaging (DTI) for visualization and quantification of the ACL graft and supports its potential to serve as a biomarker to assess graft maturity.
Introduction
Anterior cruciate ligament (ACL) reconstruction using a free (hamstring) tendon graft is one of the most common surgeries in orthopedics. It is the gold standard of care for ACL injuries, especially for young individuals and athletes who aim to return to high-level sporting activities.1 Once placed in the knee joint, the tendon graft undergoes a biologic transition from tendinous to ligamentous in appearance, a process called 'ligamentization'.2 Despite substantial research efforts, relatively little is known about the ligamentization process in the human knee. Much of the current knowledge on human graft ligamentization have been derived from biopsy studies. However, surgical biopsy procedures are invasive and may suffer from sampling error.2 Thus, a sensitive noninvasive method that can quantitatively monitor the healing process of the ACL graft is highly needed to better guide the patient’s rehabilitation and determine the appropriate timing to return to sport. Diffusion tensor imaging (DTI) is a potential candidate for this purpose. It allows for noninvasive in vivo quantification of the diffusion of water molecules inside biological tissues and assessment of its directional anisotropy, thereby providing a proxy measure of microstructural integrity.3 The purpose of this study was to demonstrate the feasibility and reliability of DTI as a tool for the 3D delineation and quantification of the ACL graft.Materials and methods
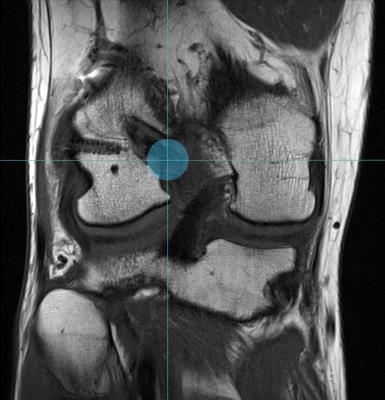
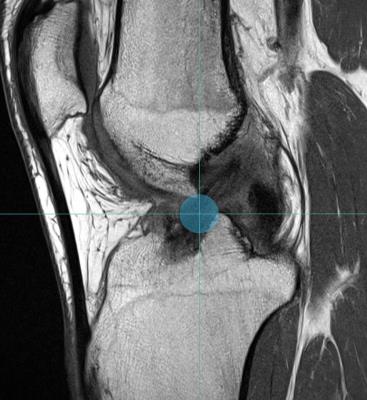
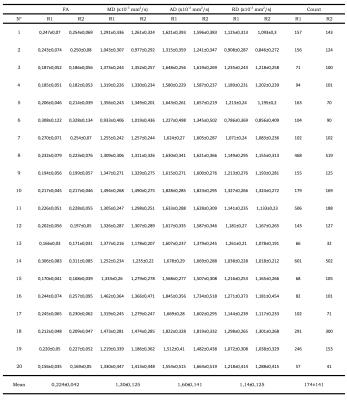
Axial diffusion-weighted EPI sequence (b = 0, 400 and 800 s/mm2 in 1, 10, and 10 directions, respectively, repeated 16 times for a total of 336 diffusion weighted volumes; TR/TE: 1300/45 ms, voxel size: 1.5×1.5×6.0 mm3, matrix: 128 × 128, TA: 7m25sec) of the knee was performed at 3T (Magnetom PrismaFit, Siemens Medical Solutions) in 20 patients within 1 year after ACL reconstruction. Multiplanar turbo spin-echo (TSE) images were also acquired to serve as anatomical reference. Tractography was performed by 2 independent radiologists to delineate the ACL graft using the following parameters: step size, 0.15mm; maximum angle, 2°; and fractional anisotropy (FA) threshold, 0.1. To isolate the ACL graft, a spherical region-of-interest (ROI) of 10mm diameter was placed both at the femoral and at the tibial apertures of the bone tunnels on the anatomical images (Figs. 1-2). These ROIs were used as both seed and target regions for the diffusion tensor tractography algorithm. The resulting fiber tractogram was converted to a track density image, which was automatically thresholded to obtain a binary mask of the ACL graft.4 Within this mask, the average FA, mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD) were extracted to obtain quantitative diffusion metrics specifically for the ACL graft. All fiber tracking operations were performed using MRtrix 0.3.15.5 Interrater reliability was assessed using the intraclass correlation coefficient (ICC) and the scan-rescan reproducibility was evaluated based on the percentage coefficient of variance (%CV) across 20 repetition bootknife samples.6Results
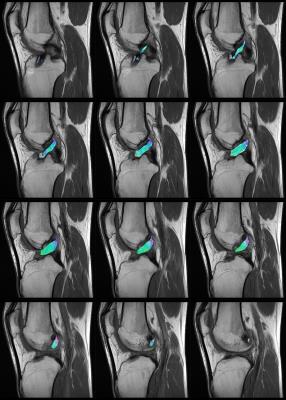
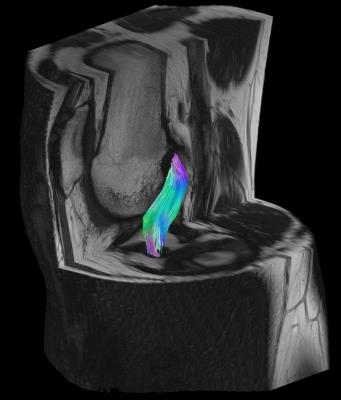
In all subjects, fiber tractography of the ACL graft was feasible with fibers of the graft that could be followed from the femoral to the tibial bone tunnel showing normal course within the intercondylar notch as perceptible on corresponding anatomical sequences (Figs. 3-4). Quantitative evaluation of the obtained fiber tracks yielded the following mean ± SD values across the population (averaged across raters): FA=0,224 ± 0,042; MD=1,30± 0,125 x 10-3 mm2/s; AD=1,60± 0,141 x 10-3 mm2/s and RD=1,14± 0,125 x 10-3 mm2/s (Table 1). Interrater reliability was excellent (ICC for FA, MD, AD and RD of 0.981, 0.907, 0.911 and 0.915, respectively). Mean number of voxels from which DTI parameters were measured was 174±141 (ICC 0.885). The scan-rescan reproducibility of ACL graft DTI metrics was high. Mean CVs (%) across all subjects for FA, MD, AD and RD were 4.6% (range 1.8%-8.7%), 4.0% (range 0.9%-11.1%), 3.6% (range 1.0%-10.4%) and 4.3% (range 1.2%-11.8%), respectively.
Conclusion
Our study demonstrates the feasibility of DTI as a tool for the 3D delineation of the ACL graft within a scan time feasible for routine clinical practice. The study also showed that the DTI derived quantitative metrics of the ACL graft are reliable and reproducible. These findings support the potential of DTI to serve as an objective biomarker to better characterize the ACL graft healing process.Acknowledgements
Pieter Van Dyck is a senior clinical investigator of the Research Foundation Flanders Belgium (FWO: 1831217N). Ben Jeurissen is a postdoctoral fellow of the Research Foundation Flanders Belgium (FWO: 12M3116N).References
1. Murawski CD, van Eck CF, Irrgang JJ, Tashman S, Fu FH. Operative treatment of primary
anterior cruciate ligament rupture in adults. J Bone Joint Surg Am. 2014;96:685–694.
2. Janssen RP, Scheffler SU. Intra-articular remodelling of hamstring tendon grafts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2014;22:2102-2108.
3. Basser PJ, Mattiello J, LeBihan D. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648.
4. Ridgway GR, Omar R, Ourselin S, Hill DL, Warren JD, Fox NC. Issues with threshold masking in voxel-based morphometry of atrophied brains. NeuroImage. 2009;44:99-111.
5. Tournier J-D, Calamante F, Connelly A. MRtrix: Diffusion tractography in crossing fiber regions. Int J Imaging Syst Technol. 2012;22:53-66.
6. Chung, Lu Y, Henry RG. Comparison of bootstrap approaches for estimation of uncertainties of DTI parameters. Neuroimage. 2006;33:531–541.
Figures