0368
Bayesian prediction for insufficient liver enhancement in gadoxetic acid-enhanced hepatobiliary phase imaging1Department of Radiology, University of Yamanashi, Chuo, Japan
Synopsis
Insufficient liver enhancement due to decreased liver function is a major limitation in gadoxetic acid-enhanced hepatobiliary phase imaging (HBP). Recent research shows that insufficient liver enhancement is associated with liver function tests including total bilirubin level, Child-Pugh classifications, indocyanine green tests, and liver stiffness measured by MR elastography. However, none of these tests have been practically used for determining the patients with insufficient liver enhancement before MR imaging. We used univariate tests and logistic regression to determine predictive factors and performed cross validation to reveal utility of Bayesian method for predicting patients with insufficient liver enhancement in gadoxetic acid-enhanced HBP.
Purpose
Gadoxetic acid or Gd-EOB-DTPA is a hepatobiliary MR contrast agent which is typically used for clinical liver MR imaging. A practical manual of liver cancer in Japan also recommends using gadoxetic acid for staging/screening of hepatocellular carcinoma1,2. However, patients with decreased liver function often show insufficient liver enhancement on hepatobiliary phase (HBP) images which obscure small lesions in the liver. Recent research shows that insufficient liver enhancement is associated with liver function tests of the patients3,4 including total bilirubin level, Child-Pugh classifications, indocyanine green clearance tests5, and liver stiffness measured by MR elastography (MRE). However, none of these tests have been practically used for determining the patients with insufficient liver enhancement in hepatobiliary phase. Bayesian prediction is a method of statistical inference in which Bayes’ theorem is used to update the posterior probability for a hypothesis as additional test result becomes available. Hence, the purpose of this study was to reveal feasibility and utility of Bayesian method for predicting patients with insufficient liver enhancement in gadoxetic acid-enhanced HBP imaging.Methods
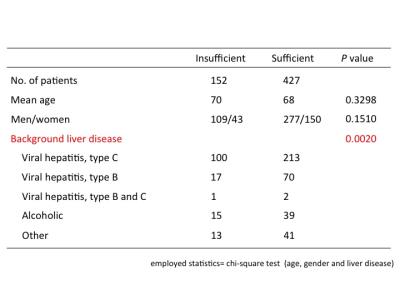
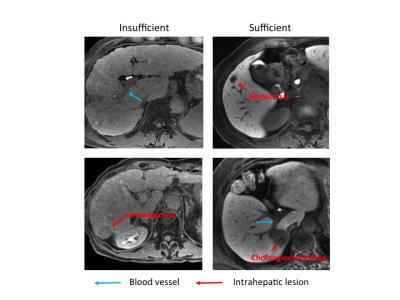
This study was performed in accordance with the principles of the Declaration of Helsinki6, and was approved by the institutional review board. 2068 patients who had undergone MRE and gadoxetic acid-enhanced MR imaging from June 2012 to December 2015 were reviewed. They were excluded if (i) all blood test results within 2 weeks were unavailable, (ii) spleen was removed, (iii) γ-GT increased abnormally due to biliary disease. We finally included 579 patients who matched these criteria (Fig. 1; Table 1). MRI was performed using a 3.0T MR unite and a 32-channel phased-array coil. We used the hepatocyte-phase images obtained 20 min after the injections. The patients were divided into two groups according to liver-to-portal contrast ratio (LPR) in HBP (sufficient and insufficient enhancement) using a cut-off value of 1.57; this cut-off value was determined by visual assessment of focal liver lesions7 (Fig.2). The numbers of patients with sufficient and insufficient liver enhancement were 427 and 152, respectively. Continuous variables of serum level of albumin (Alb), total bilirubin (T-Bil), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl transferase (γ-GT), blood platelet count (Plt), prothrombin time (PT-INR), and MRE were compared using Student’s t-test. Categorical variables of age, sex and background disease were compared using the chi-squared test. We used student’s t-test and logistic regression analysis to determine predictive factors. The feasibility of Bayesian prediction was tested by using randomly selected subjects for making basic distribution and the other subjects for validation. Data analysis was performed using python version 2.7 of the Python software (Python Software Foundation, Wolfeboro Falls, NH, USA) using the Scikit-learn library8.Results
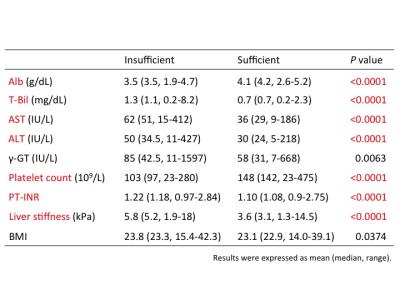
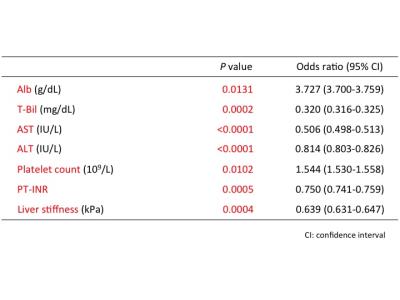
Student’s t-test analysis showed that a significant difference between the two groups was observed in serum Alb (median [range] of patients with insufficient liver enhancement vs. with sufficient liver enhancement, 3.5 [1.9–4.7] vs. 4.2 [2.6–5.2]; p=9.44e-34), T-Bil (1.1 [0.2–8.2] vs. 0.7 [0.2–2.3]; p=2.40e-22), AST (51 [15–412] vs. 29 [9–186]; p=4.54e-18), ALT (34.5 [11–427] vs. 24 [5–218]; p=3.20e-09), Plt (97 [23–280] vs. 142 [23–475]; p=3.53e-14), PT-INR (1.18 [0.97–2.84] vs. 1.08 [0.9–2.75]; p=6.91e-15) and the liver stiffness (5.2 [1.9–18] vs. 3.1 [1.3–14.5]; p=1.44e-25) (Table 2). Logistic regression analysis revealed following factors as independent associates of insufficient liver enhancement in HBP; Alb (odds ratio [OR]=3.727, 95% CI : 3.700-3.759, p=1.31e-02), T-Bil (OR=0.320, 95% CI : 0.316-0.325, p=2.11e-04), AST (OR=0.506, 95% CI : 0.498-0.513, p=6.21e-06), ALT (OR=0.814, 95% CI : 0.803-0.826, p=3.66e-05), Plt (OR=1.544, 95% CI : 1.530-1.558, p=1.02e-02), PT-INR (OR=0.750, 95% CI : 0.741-0.759, p=5.48e-04), and the liver stiffness (OR=0.639, 95% CI : 0.631-0.647, p=3.82e-04) (Table 3). The accuracy of Bayesian methods for predicting insufficient liver enhancement was 78.9% by Alb only, 78.2% by T-Bil only, 73.7% by Plt only and 76.0% by PT-INR only. However, the accuracy became 82.4% by combining Alb, T-Bil, Plt and PT-INR.Conclusion
There are many indicators of insufficient enhancement9-16as previously studied. Bayesian prediction is feasible and implementable in clinical setting for the sake of predicting patients with insufficient enhancement in HBP. By combining more than one factors using Bayesian methods, more precise prediction was available.Acknowledgements
No acknowledgement found.References
1.Hammerstingl R, Huppertz A, Breuer J, et al. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol 2008;18:457–467. 2.Bluemke DA, Sahani D, Amendola M, et al. Efficacy and safety of MR imaging with liver-specific contrast agent: U.S. multicenter phase III study. Radiology 2005;237:89–98. 3.Tschirch FT, Struwe A, Petrowsky H, Kakales I, Marincek B, Weishaupt D. Contrast-enhanced MR cholangiography with Gd-EOB-DTPA in patients with liver cirrhosis: visualization of the biliary ducts in comparison with patients with normal liver parenchyma. Eur Radiol 2008;18:1577–1586. 4.Ryeom HK, Kim SH, Kim JY, et al. Quantitative evaluation of liver function with MRI Using Gd-EOB-DTPA. Korean J Radiol 2004;5:231–239. 5.Motosugi, U. et al. Liver parenchymal enhancement of hepatocyte-phase images in Gd-EOB-DTPA-enhanced MR imaging: which biological markers of the liver function affect the enhancement? J Magn Reson Imaging 2009;30:1042–1046. 6. World Medical Association Declaration of Helsinki.Ethical principles for medical research involving human subjects. Bull World Health Organ 2001;79:373–374. 7. Motosugi U, Ichikawa T, Tominaga L, et al. Delay before the hepatocyte phase of Gd-EOB-DTPA-enhanced MR imaging: is it possible to shorten the examination time? Eur Radiol 2009 [Epub ahead of print]. 8. Pedregosa, F., Varoquaux, G., Gramfort A., Michel V., Thirion B., Grisel, O., Blondel, M., Prettenhofer, P., Weiss, R., Dubourg, V., Vanderplas, J., Passos, A., Cournapeau, D., Brucher, M., Perrot, M., and Duchesnay, E.: Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011;12:2825-2830. 9. Kim HY, Choi JY, Park CH, Song MJ, Song do S, Kim CW, Bae SH, Yoon SK, Lee YJ, Rha SE. Clinical factors predictive of insufficient liver enhancement on the hepatocyte-phase of Gd-EOB-DTPA-enhanced magnetic resonance imaging in patients with liver cirrhosis. J Gastroenterol. 2013;48(10):1180-7. 10. Okada M, Ishii K, Numata K, Hyodo T, Kumano S, Kitano M, Kudo M, Murakami T. Can the biliary enhancement of Gd-EOB-DTPA predict the degree of liver function? Hepatobiliary Pancreat Dis Int. 2012;11(3):307-13. 11. Motosugi U, Ichikawa T, Muhi A, Sano K, Morisaka H, Ichikawa S, Araki T. Magnetic resonance elastography as a predictor of insufficient liver enhancement on gadoxetic acid-enhanced hepatocyte-phase magnetic resonance imaging in patients with type C hepatitis and Child-Pugh class A disease. Invest Radiol. 2012;47(10):566-70. 12. Lee S, Choi D, Jeong WK. Hepatic enhancement of Gd-EOB-DTPA-enhanced 3 Tesla MR imaging: Assessing severity of liver cirrhosis. J Magn Reson Imaging. 2016;44(5):1339-1345. 13. Tschirch FT, Struwe A, Petrowsky H, Kakales I, Marincek B, Weishaupt D. Contrast-enhanced MR cholangiography with Gd-EOB-DTPA in patients with liver cirrhosis: visualization of the biliary ducts in comparison with patients with normal liver parenchyma. Eur Radiol. 2008;18(8):1577-86. 14. Kukuk GM, Schaefer SG, Fimmers R, Hadizadeh DR, Ezziddin S, Spengler U, Schild HH, Willinek WA. Hepatobiliary magnetic resonance imaging in patients with liver disease: correlation of liver enhancement with biochemical liver function tests. Eur Radiol. 2014;24(10):2482-90. 15. Matsushima S, Sato Y, Yamaura H, Kato M, Kinosada Y, Era S, Takahashi K, Inaba Y. Visualization of liver uptake function using the uptake contrast-enhanced ratio in hepatobiliary phase imaging. Magn Reson Imaging. 2014;32(6):654-9. 16. Talakic E, Steiner J, Kalmar P, Lutfi A, Quehenberger F, Reiter U, Fuchsjäger M, Schöllnast H. Gd-EOB-DTPA enhanced MRI of the liver: correlation of relative hepatic enhancement, relative renal enhancement, and liver to kidneys enhancement ratio with serum hepatic enzyme levels and eGFR. Eur J Radiol. 2014;83(4):607-11. 17. Ali Nassif, Jia Jia, Markus Keiser, Stefan Oswald, Christiane Modess, Stefan Nagel, Werner Weitschies, Norbert Hosten, Werner Siegmund, Jens-Peter Kühn. Visualization of Hepatic Uptake Transporter Function in Healthy Subjects by Using Gadoxetic Acid–enhanced MR Imaging. Radiology. 2012;264(3):741-50Figures