0361
Robust Visualization of MCA Main Trunk by Improved Acceleration-Selective Arterial Spin Labeling (iAccASL) for Intracranial MR Angiography1Philips Electronics Japan, Shinagawa, Tokyo, Japan, 2Department of Clinical Radiology, Graduate School of Medical Science, Kyushu University, Fukuoka, Japan, 3Department of Radiology, Tokai University Hospital, Japan, 4Asia Pacific, Philips Healthcare, Shinagawa, Tokyo, Japan
Synopsis
Improved acceleration-selective arterial spin labelling (iAccASL) was implemented for Intracranial MR angiography and images were acquired in six healthy volunteers. Additional 180゜refocusing pulses in control module will efficiently suppress spin dispersion and flow voids in strongly accelerating middle cerebral artery (MCA) main arteries. The vessel visualizations of MCA main trunk and peripheral vessels were compared with our previously reported AccASL and conventional time-of-flight (TOF) approach. We demonstrated iAccASL could produce better MCA main trunk visualization (without venous signal) compared with conventional AccASL technique with better peripheral visualization than TOF.
INTRODUCTION
The three dimensional time of flight (3D-TOF) acquisition is the standard for routine clinical intracranial MR angiography (MRA).1-4 However, TOF has several limitations, such as reduced visualization of flow that is not in the feet to head direction or slow flow, due to diminished inflow effect.
To overcome the limitations of TOF, last year, the acceleration sensitized arterial spin labelling (ASL) based technique, named acceleration-selective ASL (AccASL), was proposed.5 Since AccASL directly labels spins of target vessels; this approach is not sensitive to inflow effects. In addition, because arterial blood motion has a major accelerating component, AccASL is a highly artery selective labelling approach. However, AccASL has some limitations in visualizing the main trunk of the middle cerebral artery (MCA). We hypothesize that this problem is caused by spin dispersion and by flow voids in the strongly accelerating main trunk of MCA during the control module.
To deal with the problem, we increased the number of refocusing pulses in the control module to try to suppress spin phase dispersion in the MCA main trunk. We name this modified sequence improved AccASL (iAccASL). In this work, we implemented iAccASL and validated its clinical efficacy by comparing iAccASL with AccASL and conventional TOF.
METHODS
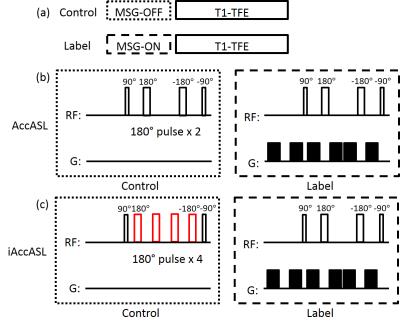
iAccASL scheme: The iAccASL and AccASL sequences consist of a control (T2-preparation without MSG) or label (with MSG) module followed by a T1-weighted turbo field echo (TFE) read-out, as shown in Fig. 1a. The T2-preparation consists of a 90゜excitation pulse followed by MLEV 180゜refocusing pulses 6 and a 90゜flip back pulse. The number of 180゜refocusing pulses in the control module is two for AccASL and four for iAccASL (Fig. 1b). The MSG strength is defined by the acceleration causing a phase change of π (AENC: acceleration-encoding).
Subject and equipment: This sequence was implemented on a 3.0-T MR scanner (Philips, Ingenia R5.17). Six healthy volunteers were examined after obtaining informed consent as required by hospital review board. iAccASL, AccASL and TOF images were acquired for image comparison.
Sequence parameters: Acquisition parameters of iAccASL and AccASL were: sequence T1-TFE, TR/TE 8.5/2.7ms, FA 11°, ETL 60, SPIR, 3D slab thickness 120mm, voxel size 0.5*1.0*1.2 (100 partitions), SENSE factor 2.3, AENC 2.31m/s2, total acquisition time (label and control) 4m39s. TOF acquisition parameters were: sequence T1-FFE, TR/TE 23/3.5ms, FA 18°, acquisition time 4m37s. Geometry parameters, 3D slab thickness, voxel size and SENSE factor of TOF were same as iAccASL and AccASL.
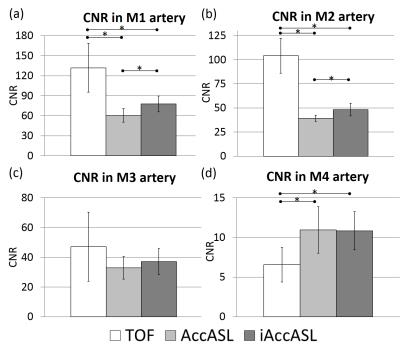
Assessment of CNR in MCA: Analysis of CNRs of M1~M4 of MCA was performed in six healthy volunteers. 25mm slab thickness Maximum intensity projections (MIPs) were generated. Circular ROIs were placed on white matter (WM) and the middle of the M1~M3 arteries. The ROI for the M4 artery was placed at its peripheral, as observable in the TOF image. The contrast-to-noise ratios (CNRs) between each of M1~M4 arteries and WM were calculated as CNR = (bloodmax - WMave)/WMSD, where bloodmax is the maximum signal intensity in the MCA ROIs. The average CNRs of the left and right MCA were compared among three sequences. The Holm method was used for the statistical analysis and a p value less than 0.05 was considered significant.
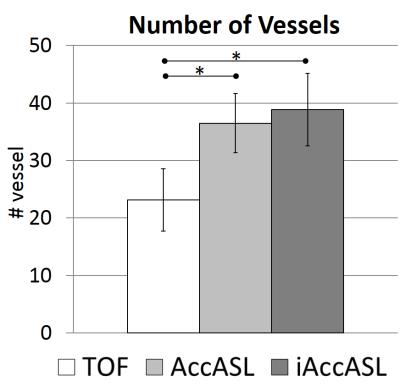
Assessment of vessel visualization: Straight lines were drawn in the MCA area and line profiles were obtained. We counted the number of vessels, with a vessel defined as observable if Vessel signal > WMave + 5*WMSD. In addition, a radiologist with sixteen years of experience in neuroradiology (OT) and an MR scientist with fourteen years of experience (MO) assessed the cortical vein signal.
RESULTS
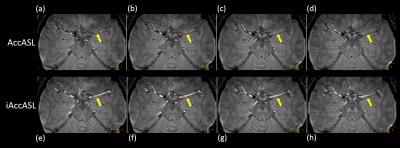
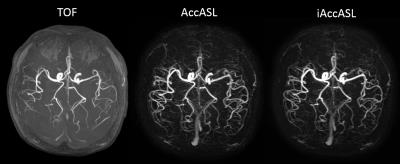
Representative axial consecutive images in MCA area in control module are shown in Figs. 2. In the MCA main trunk more intense arterial signals, without flow voids, were observed in iAccASL compared to AccASL (yellow arrows). Visualization of peripheral vessels of AccASL and iAccASL images was better than that of TOF (Fig. 3). In Figs. 4, CNRs in M1 and M2 for iAccASL are significantly higher than those of AccASL; M1 (p=0.00077), M2 (p=0.00971). In addition, CNRs in M4 for AccASL and iAccASL are significantly higher than that of TOF: AccASL (p=0.022), iAccASL (p=0.0078). In Fig. 5, vessel numbers for AccASL and iAccASL are significantly higher than that of TOF: AccASL (p=0.0076), iAccASL (p=0.0027). For the six volunteers checked by the radiologist and the MR scientist, cortical vein signal that could affect clinical diagnosis was not observed.CONCLUSION
We have demonstrated 3D intracranial MRA with iAccASL technique. These results indicate that iAccASL can provide better MCA main trunk visualization compared with conventional AccASL technique with better peripheral visualization than TOF.Acknowledgements
No acknowledgement found.References
1. Wong EC et al, MRM 2006;55:1334
2. Kimura T et al, MRM 2009;62:450
3. Schmid S et al, MRM 2014;71:191
4. Priest AN et al, MRM 2014;72:699,
5. Obara M et al, MRM 2016 (DOI: 10.1002/mrm.26275)
6. Levitt M et al, JMR 1982;47:328
Figures