0358
Whole-Brain Arteriography and Venography Using an Improved Velocity-Selective Saturation (VSS) Pulse Trains1Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3Developing Brain Research Lab, Children’s National Medical Center, Washington, DC, United States, 4Department of Radiology, People’s Hospital, Guangzhou, People's Republic of China, 5Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland, Baltimore, MD, United States
Synopsis
Velocity-selective (VS) MRA, a non-subtractive technique and allows large spatial coverage and slow-flow depiction has shown recent promise for cerebral applications at 3T. Here, we improved the velocity-selective saturation (VSS) pulse train by reducing its sensitivity of tissue suppression to B1 inhomogeneity for both the intracranial and cervical regions. Moreover, we propose that arteriograms or venograms can be obtained by placing spatially selective inversion pulses before the acquisition to selectively null signals from venous or arterial blood. The feasibility of these technical advances for VS MRA is demonstrated on healthy volunteers at 3T.
Introduction
Time-of-flight (TOF)1 is the most established non-contrast-enhanced (NCE) magnetic resonance angiography (MRA) technique with flexible acquisition options for arteriography and venography, but suffers from limited spatial coverage and poor delineation of slow flow2. Arterial spin labeling (ASL)3–5 and phase contrast (PC)6 MRA provide large spatial coverage, but are limited by longer scan times due to paired acquisitions for subtraction and an incapability of generating venography. The emerging velocity-selective (VS) MRA technique allows for large angiographic (spatial) coverage and slow-flow sensitivity using a single acquisition without subtraction7,8. Here we aim to develop a 3D NCE MRA method that allows arteriography and venography with whole-brain coverage, using a newly constructed velocity-selective saturation (VSS) pulse trains for improved immunity to B1 inhomogeneity.Methods
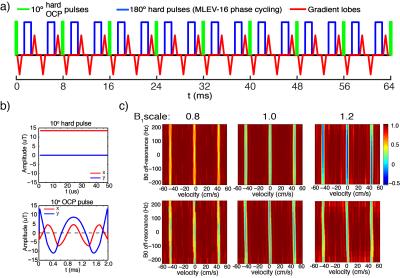
The VSS pulse train is composed of a series of 9 excitation pulses (10° each), interleaved with pairs of 180° refocusing hard pulses and velocity encoding gradient lobes (Fig. 1a). The previous use of hard pulses7 for excitation causes the saturation band to be sensitive to B1 inhomogeneity, leading to an inhomogeneous saturation of background tissue. To tackle this issue, a 10° optimized composite (OCP) pulse (~2ms, Fig. 1b) was generated using the optimal control method9. Fig. 1c displays the Mz responses of VSS pulse trains employing hard pulses (the first row) and OCP pulses (the second row) over the plane of velocity (x-axis) vs. B0 off-resonance (y-axis) at three different B1+ scales. This substitution with 10° OCP pulses significantly mitigated the dependence of the tissue saturation on the B1 inhomogeneity.
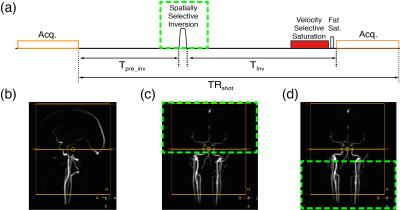
In order to obtain an arteriogram and venogram, a spatially-selective inversion pulse is applied with a delay time (Tinv) inserted before the VSS pulse train (Fig. 2a). At each acquisition, the inverted magnetization of downstream venous blood or upstream arterial blood recovers to the nulling point, resulting in an arteriogram or venogram, respectively. For instance, with head-neck angiographic coverage (Fig. 2b), the inversion pulse is applied to the entire intracranial region for arteriography (green dashed box, Fig. 2c) and the cervical region for venography (green dashed box, Fig. 2d). Since the T1 of blood at 3T is about 1.8 s 10,11 and the transit times from the carotid to intracranial vessels range from 0.5 to 1.2 s 5, the calculated inversion nulling time (~0.9 s) allows for fill-in of fresh or nulled arterial blood for arteriography or venography, respectively.
3D angiograms, arteriograms and venograms of 6 healthy volunteers (36+/-9 y, 3M, 3F) were obtained on a 3T Philips Achieva scanner (body coil transmission and 32-channel head coil reception). A multi-shot turbo field echo (TFE) with low-high profile ordering was used for acquisition with axial orientation: FOV, 180×180×220 mm3; acquired resolution, 0.7×0.7×1.4 mm3; TR/TE, 7.5/2.4 ms; flip angle, 7°; SENSE factor of 2×2; total scan time, 5.2 min for each angiogram. The velocity-encoding gradients of the VSS pulse trains were applied along the foot-head direction. Angiograms with 10° hard excitation pulses in VSS pulse trains were obtained for comparison. The standard TOF arteriograms obtained with multi-chunks and ramped excitation were also acquired with identical resolution, but smaller superior-inferior coverage (80 mm).
Results
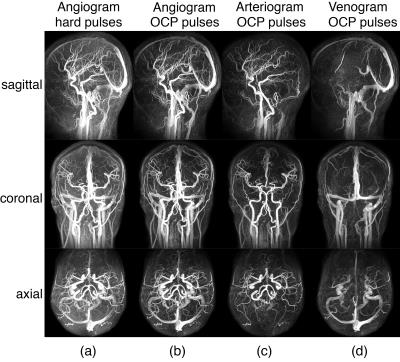
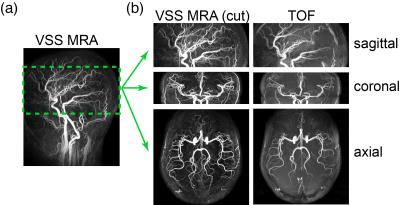
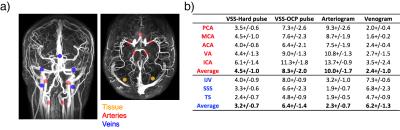
The MIP images of VSS-MRA angiograms acquired with 10° hard pulses and OCP pulses are shown in Fig. 3a and Fig. 3b, respectively. Tissue background is more uniformly suppressed when OCP pulses are used compared with hard pulses. The arteriograms (Fig. 3c) depict all the major cervical and intracranial arteries and some small branches. The venograms (Fig. 3d) delineate all the major intracranial and cervical veins. Compared to TOF, the extracted slabs from the VSS arteriograms better depict small distal branches with slow in-plane flow (Fig. 4). The averaged contrast ratios (Signalblood /Signaltissue) of major arterial and venous ROIs in VSS-MRA on 6 subjects are reported in Fig. 5. The averaged contrast ratios of VSS-MRA using OCP pulses are nearly twice as high as those obtained using hard pulses in arteries (8.3 vs 4.5) and veins (6.4 vs 3.2). The arteriograms yield more than 4 times higher contrast ratios in arteries than veins (10.0 vs 2.3) while the venograms have almost 3 times the contrast ratios of venous blood compared to arterial blood (6.2 vs 2.4).Conclusion
We have improved VSS pulse trains by reducing its B1 sensitivity and developing protocols for 3D arteriography and venography. The high-resolution whole-brain angiograms acquired in ~5min will be further investigated in patients with cerebrovascular disorders.Acknowledgements
We thanks Joseph Gillen for helpful discussion.References
1. Dumoulin CL, Cline HE, Souza SP, Wagle WA, Walker MF. Three-dimensional time-of-flight magnetic resonance angiography using spin saturation. Magn. Reson. Med. 1989;11:35–46.
2. Axel L. Blood flow effects in magnetic resonance imaging. Am. J. Roentgenol. 1984;143:1157–1166.
3. Wu H, Block WF, Turski PA, Mistretta CA, Rusinak DJ, Wu Y, Johnson KM. Noncontrast dynamic 3D intracranial MR angiography using pseudo-continuous arterial spin labeling (PCASL) and accelerated 3D radial acquisition. J. Magn. Reson. Imaging 2014;39:1320–1326.
4. Tan ET, Huston J, Campeau NG, Riederer SJ. Fast inversion recovery magnetic resonance angiography of the intracranial arteries. Magn. Reson. Med. 2010;63:1648–1658.
5. Robson PM, Dai W, Shankaranarayanan A, Rofsky NM, Alsop DC. Time-resolved vessel-selective digital subtraction MR angiography of the cerebral vasculature with arterial spin labeling. Radiology 2010;257:507–515.
6. Dumoulin CL, Souza SP, Walker MF, Wagle W. Three-dimensional phase contrast angiography. Magn. Reson. Med. 1989;9:139–149.
7. Qin Q, Shin T, Schär M, Guo H, Chen H, Qiao Y. Velocity-selective magnetization-prepared non-contrast-enhanced cerebral MR angiography at 3 Tesla: Improved immunity to B0/B1 inhomogeneity. Magn. Reson. Med. 2016;75:1232–41.
8. Shin T, Qin Q, Park JY, Crawford RS, Rajagopalan S. Identification and reduction of image artifacts in non-contrast-enhanced velocity-selective peripheral angiography at 3T. Magn. Reson. Med. 2016;76:466.
9. Liu H, Matson GB. Radiofrequency pulse designs for three-dimensional MRI providing uniform tipping in inhomogeneous B1 fields. Magn. Reson. Med. 2011;66:1254–1266.
10. Li W, Liu P, Lu H, Strouse JJ, van Zijl PCM, Qin Q. Fast measurement of blood T1 in the human carotid artery at 3T: Accuracy, precision, and reproducibility. Magn. Reson. Med.:Epub ahead.
11. Qin Q, Strouse JJ, van Zijl PCM. Fast measurement of blood T1 in the human jugular vein at 3 Tesla. Magn. Reson. Med. 2011;65:1297–304.
Figures