0356
Simultaneous cerebral blood flow and bold oxygen level dependent signal assessments using multi-band multi-echo pseudo-continuous arterial spin labeling (M2-PCASL)1Georgia Institute of Technology, Atlanta, GA, United States, 2Department of Radiology and Imaging Sciences, Emory University, Atlanta, GA, United States, 3MR R&D Collaborations, Siemens Healthcare, Atlanta, GA, United States, 4Department of Neurology, Emory University, Atlanta, GA, United States
Synopsis
We proposed a novel multi-band multi-echo pseudo-continuous arterial spin labeling (M2-PCASL) method to simultaneously measure the cerebral blood flow (CBF) and the blood oxygen level dependent (BOLD) signal. Increased spatial resolution was achieved compared to the conventional method, and experiments were also conducted to show simultaneous measurement of CBF and BOLD signal dynamics in response to hypercapnia. M2-PCASL can be a useful tool for measuring cerebrovascular reactivity and studying neurovascular coupling in various conditions.
Introduction
Measuring cerebrovascular reactivity (CVR) allows stratifying risk of stroke among patients with cerebrovascular steno-occlusive diseases1, and could be a useful measurement in evaluating the role of cerebrovascular contribution to aging and Alzheimer’s disease. Multi-echo pseudo-continuous arterial spin labeling (PCASL) sequence was previously used to simultaneously estimate cerebral blood flow (CBF) and blood oxygen level dependent (BOLD) signal under hypercapnia2, which, however, suffers from limited spatial coverage due to increased readout time of multiple echoes in combination with short longitudinal relaxation time of the magnetically tagged blood. In the present study, we proposed a novel multi-band multi-echo PCASL (M2-PCASL) sequence to increase spatial resolution of both CBF and BOLD assessment. Experiments were conducted to show CBF and BOLD values and the changes in these values under hypercapnia.Methods
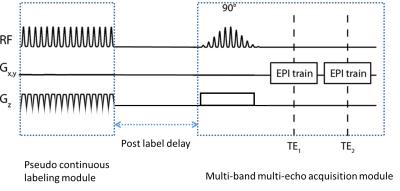
M2-PCASL sequence was designed and implemented on a Siemens platform, which includes modules for pseudo-continuous labeling of blood flow, multiband excitation of multiple slices, and multi-echo EPI acquisition (Figure 1). The sequence was initially tested on a phantom. Four healthy volunteers (age: 20.5±1.9 years; 3 Females and 1 male) were recruited to undergo MRI scans using M2-PCASL sequence for the measurement of CBF and BOLD, and the obtained CBF values were compared with conventional PCASL results. The hypercapnia challenges through breathing air with increased CO2 (8%) mixture and/or breath-holding were performed, which were known to induce increased CBF and BOLD signal in healthy subjects. The scans were performed at a Siemens Tim Trio 3T scanner with a 32-channel head coil using M2-PCASL sequence. The scan parameters were as follows: TR =4.7955 s, TE = 12/27.7 ms, FOV = 220 x 220 mm2, matrix = 72x72, slice thickness/gap = 3.10/0.31 mm, total number of slices = 36, total number of time frames = 80, multiband factor = 2, GRAPPA factor =2; for the PCASL module, a labelling duration of 1.472, a post-label delay of 1 second, and a pre-label delay of 0.9 second due to limitations of specific absorption rate were used. During the hypercapnia experiments, following a two-minute baseline scan when the subjects were breathing room air, the breathing gas was switched to 8% CO2 mixture for 2 minutes, followed by room air breathing for the rest of the scan. During the scan end-tidal CO2 concentration was monitored using a BioPac system (GOLETA, CA).
For preprocessing, motion correction and skull stripping were performed on the images of the first echo and the derived transformation parameters and masks were applied to the images of the second echo. Time frames with head motion larger than 2mm were removed from further analysis. The multi-echo temporal signal is expressed as the following equation:
$$S(n, TE)=S_0(n)e^{-TE\cdot R_2^*(n)}+\sigma, n=1, 2, …, 80$$
where TE denotes the echo time, $$$R_2^*(n)$$$ is the transverse relativity, and σ is noise. Therefore, $$$R_2^*(n)$$$, $$$S_0(n)$$$, and BOLD signal can be estimated by the following equations:
$$R_2^*(n)=ln(S(n, TE_2)/S(n, TE_1))/(TE_1-TE_2)$$
$$S_0(n)=(S(n, TE_1) /e^{-TE_1 R_2^*(n)}+S(n, TE_2) /e^{-TE_2 R_2^*(n)})/2$$
$$BOLD(n)=S_0(n)e^{-TE_1 R_2^*(n)}$$
CBF image was calculated using the signal from the first echo according to equations from a previously published work3.
Results
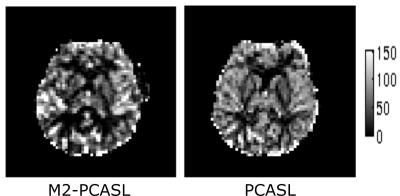
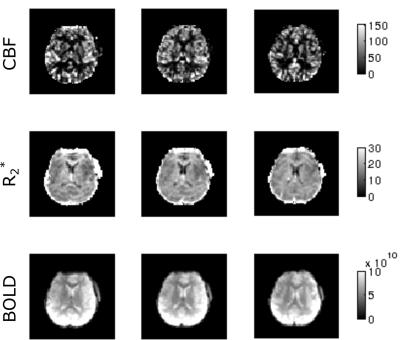
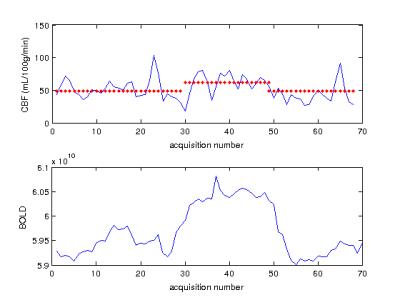
Figure 2 shows a comparison between CBF images acquired using M2-PCASL and conventional single band techniques. A spatial resolution of 3.1x3.1x3.4 mm3 with whole brain coverage was achieved using M2-PCASL while the resolution of the conventional PCASL was 3.4x3.4x7.2 mm3. Figure 3 demonstrates the CBF, R2*, and BOLD images derived from M2-PCASL. The CBF and BOLD time courses averaged across the entire brain are plotted in Figure 3. Inhalation of 8% CO2 was associated with a 1.74% increase in BOLD signal and a 27.69% increase in CBF (Figure 4). Figure 5 shows CVR map calculated as percentage BOLD signal change caused by hypercapnia. The CVR image was smoothed with 6mm FWHM Gaussian kernel for better visualization.Discussion and Conclusion
Figure 2 demonstrates that the increased spatial resolution afforded by M2-PCASL alleviates partial volume effects. Figure 4 shows a clear increase in both CBF and BOLD under hypercapnia condition. Although the percentage increase in CBF is larger than that of BOLD signal, it also suffers from more fluctuations caused by physiological noise, which is consistent with literature2. Potentially, we can also increase the temporal resolution of M2-PCASL, which would allow for observation of more subtle temporal changes in CBF and BOLD.
In conclusion, we have proposed a novel M2-PCASL sequence that allows simultaneous measurement of CBF and BOLD signal with increased spatial resolution, which could provide a useful tool in evaluating cerebrovascular hemodynamic functions of cerebrovascular steno-occlusive disease or Alzheimer’s disease patients.
Acknowledgements
No acknowledgement found.References
1. Gupta, A. et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion a systematic review and meta-analysis. Stroke 43, 2884-2891 (2012).
2. Zhou, Y., Rodgers, Z. B. & Kuo, A. H. Cerebrovascular reactivity measured with arterial spin labeling and blood oxygen level dependent techniques. Magnetic resonance imaging 33, 566-576 (2015).
3. Qiu, D. et al. CBF measurements using multidelay pseudocontinuous and velocity-selective arterial spin labeling in patients with long arterial transit delays: Comparison with xenon CT CBF. Journal of Magnetic Resonance Imaging 36, 110-119 (2012).
Figures