0354
A Novel Approach to Measuring Cerebral Oxygen Extraction Fraction and Vascular Reserve Using MRICharles Cantrell1, Yong Jeong2, Kevin Midlash3, Parmede Vakil2, Sameer Ansari2, and Timothy J Carroll3
1Northwestern University, Chicago, IL, United States, 2Northwestern University, 3University of Chicago
Synopsis
The length scales associated with parenchymal oxygen extraction fraction (OEF), coupled with the near uniformity of normative OEF across the brain dictate the development of an imaging approach that is sensitive to low-spatial frequency imaging behavior. Previous approaches to measure OEF using MRI have utilized high pass-filters—effectively removing much of the signal. We propose a new method to filter out geometric field inhomogeneity, by imaging temporally through the cardiac cycle. In a study in 11 patients with intracranial atherosclerotic disease, we found elevated OEF on the compromised hemisphere as compared to the healthy contralateral side (p<0.0195).
Introduction
Over the past 20 years, considerable work has been done to understand the brain’s auto-regulatory systems and how certain deficiencies may lead to hemodynamic failure. By quantifying compensatory mechanisms, Derdeyn found that patients with increased cerebral oxygen extraction fraction (OEF) distal to a carotid artery occlusion, were more likely to have a stroke within the next year [1]. Traditional approaches developed to measure cerebral OEF with MRI rely on the susceptibility differences between oxygenated and deoxygenated blood. The length scales associated with parenchymal OEF, coupled with the near uniformity of normative OEF across the brain dictate the development of an imaging approach that is sensitive to low-spatial frequency imaging behavior. Removal or “normalization” of low spatial frequency signal is required to mitigate air/tissue boundaries in the auditory canals and frontal sinus and other sources of non-uniform local magnetic fields. The residual phase resulting from geometric field inhomogeneity has been a challenge for the clinical translation of OEF imaging. To address this challenge, we have developed scan protocol and post processing algorithm to filter out geometric field inhomogeneity and relax the spatial frequency requirements on the susceptibility mapping used in OEF imaging. Outside the static dephasing regime, magnetic shift between oxygenated hemoglobin (oHb) and deoxygenated hemoglobin (dHb) creates a local frequency and phase shift proportional to OEF[2]: $$δω = 4/3 γ π Δx_0 * Hct * (OEF * B_0)$$ However local alterations in the main magnetic field $$$(ΔB_0 (x,y) $$$ are large (~100-200 Hz at 3.0T) and overwhelm the 15–30 Hz signal changes anticipated from theory[2]. To mitigate the effect of static field inhomogeneity, we synchronize our acquisition to the inflow of oxygenated blood and integrate the change in susceptibility over the cardiac cycle.Methods
Cerebral Oxygen Extraction Fraction: Twieg’s Parameter Assessment by Retrieval from Signal Encoding (PARSE) acquisition[3] is based on a rosette trajectory and maximum likelihood fit yields parametric images of frequency offset, R2* and M0. PARSE provides high, accurate sensitivity to local frequency shits in an 80 ms readout and is therefore ideal for OEF imaging(Figure 1). We acquire 20 single slices acquired at successive times after the cardiac QRS complex. The goal is to measure the change in frequency shift as oxygenated blood is metabolize into de-oxygenated blood. The dynamic signal changes acquired with PARSE were decomposed into d.c. components (Figure 2) which contain all the local magnetic field inhomogeneity and dynamic components which represent the change in local susceptibility which occur as oxygen is extracted from fresh blood supplied to the parenchyma: $$ δω(x,y,t) = γΔB_0(x,y) + ∫4/3 γ π Δx_0 * Hct * (OEF(x,y,t) * B_0) dt $$ Cerebral Reactivity: Dynamic components of the OEF signal (OEF(x,y,t)) were modelled a Linear Time Invariant System where the input function (i.e. heartbeat) is approximated as a unit impulse and the change in parenchymal susceptibility after the initial delivery of oxygenated blood is the system output. We define the Vascular Reactivity Function(VRF): VRF ≈ 1/Vr e(-t/V_rC) where Vr is the vascular resistance of the brain and C is the vascular compliance, characterizes the system. We define b = 1/VrC to be vascular reactivity. The PARSE acquisition consisted of a single slice, 5.0mm thick, 210mm x 210mm FOV, 108x108 matrix, resolution = 1.94x1.94x5 mm3 2D PARSE images. Prospective cardiac gated was implemented in patients to synchronize image acquisition to the inflow of oxygenated blood. Each 2D slice was acquired 25 times, at 25 ms increments from the R-trigger throughout the cardiac cycle (25 ms to 625 ms delay).Results
A series of 11 consecutive patients (M/F 5/6, <age>=52.1±11.1) referred for the evaluation of symptoms indicative of ischemic stroke or transient ischemic attack (TIA), secondary to intracranial atherosclerotic disease (ICAD). OEF in the normal hemispheres was (44%±6.7%) which is in agreement with historical reference PET-OEF and significantly increase (56 %±6.7%, p<0.0195). β values showed additional information, with hemispheric significance (10.72 ± 3.48 10-3ms-1, 9.69 ± 3.51 10-3ms-1; p<0.037).Discussion/Conclusions
In this pilot study, we present the first evidence of an MR-based OEF and CVR technique that requires no contrast. We have found that MR-PARSE has detectable sensitivity to frequency shifts induced by transient alterations in de-oxyhemoglobin through the cardiac cycle in ICAD patients with greater than 50% stenosis. Furthermore, we have shown that through the use of ICA, transient OEF and β are significant predictors of hemispheric compromise. Our approach to quantify transient BOLD fluctuations due to cerebrovascular reactivity represents a new and simple, non-contrast approach to stratifying patients toward therapies to prevent stroke.Acknowledgements
R01NS093908; R21EB01792References
[1] Derdeyn, et al Brain 2002 [2] Yablonskiy et al MRM 1994, [3] Twieg, et al MRM 2002, Menon et al, JCBFM 2012Figures
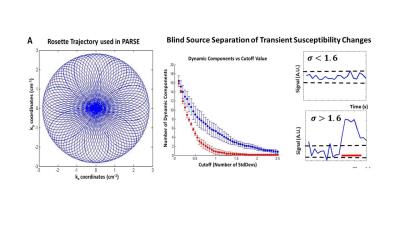
Figure 1: A
single-shot Rosette readout and PARSE image reconstruction are synchronized to
the cardiac cycle. Image reconstruction using PARSE produces a time-series of
frequency maps which in which independent component analysis is used to filter
out static magnetic field inhomogeneity. The resulting images are converted of
cerebral OEF images and parametric maps of vascular reactivity
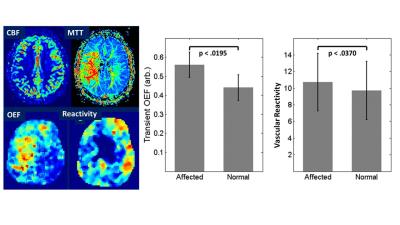
Figure 2: Parametric images of
cerebral blood flow (CBF), mean transit time (MTT) show hemispheric alteration
resulting from a high grade middle cerebral arterial stenosis. Cardiac gated
susceptibility changes show that cerebral oxygen extraction fraction (OEF) is
hemispherically increase and cerebrovascular reserve (inverse of reactivity) is
decreased. Note that OEF and reactivity
were acquired in 100 seconds without the need for contrast agent or