0352
Cerebral blood flow as a marker for cortical parcellation1Department of Cognitive Neuroscience, Maastricht University, Maastricht, Netherlands
Synopsis
Within-subject differences of T1, T2* and cortical thickness can be used to automatically parcellate the cortex, e.g. to guide functional analyses or increase statistical power. Here, we evaluate the additional benefit of baseline CBF as a marker for brain metabolism to differentiate regions. We demonstrate that CBF data is not redundant with the other quantitative MRI parameters. Therefore, a data-driven parcellation of brain regions that incorporates perfusion information allows delineation of the cortex into smaller units and enhances subsequent anatomical & functional analysis.
Purpose
Quantitative imaging parameters, such as of T1, T2* and cerebral blood flow (CBF), detect absolute differences on the brain’s microstructure and physiology within and between subjects due to learning, gender, age and disease effects. In addition, within-subject differences of these tissue characteristics can be used to parcellate the cortex into smaller regions, e.g. to guide functional and anatomical analyses or increase statistical power. A semi-automatic multi-modal parcellation framework using cortical thickness and myelin-related contrasts, such as T1w and T2w maps, was recently introduced1. Here, we evaluate the additional benefit of baseline CBF as a marker for brain metabolism to differentiate between pre-defined regions based on the FreeSurfer Destrieux cortical atlas.Methods
Subjects and MRI: Nine healthy volunteers (aged 39.7 ± 8.9, 3 males) were scanned using a whole-body 7T scanner (Siemens Medical Systems, Erlangen, Germany) and a 32-channel phased-array coil (Nova Medical, Wilmington, USA). For each subject, whole-brain submillimeter (0.7 mm3) R1 (= 1/T1) and T2* maps were acquired with TR/TE/TI1/TI2 = 5000/2.47/900/2750 ms, α1/α2 = 5°/3°, GRAPPA 3 for MP2RAGE and TE1/TE2/TE3/TE4/TR = 2.53/7.03/12.55/20.35/33 ms for multi-echo GRE sequences, respectively. Cerebral blood flow (CBF) was obtained following the ASL FAIR QUIPSS II scheme with TI1/TI2/TE/TR = 700/1800/13/2500 ms4 and 2.8 mm3 voxel-size. In addition, B1+ was mapped using the SA2RAGE sequence with TR/TE = 2400/0.78 ms, TD1/TD2 = 580/1800 ms, α1/α2 = 4°/11°, GRAPPA 2 and 3 mm3 voxel-size. Dielectric pads were placed proximal to the temporal lobes to locally increase the transmit B1+ field and to improve its homogeneity across the brain2. Analysis: First, all maps were coregistered to the MP2RAGE data using SPM8 (Wellcome Department of Imaging Neuroscience, University College London, London, UK). Next, B1+-corrected R1 maps were obtained using the code and procedure as described previously3, T2* maps were computed from the GRE data using a monoexponential fit and CBF data was computed as in Ivanov et al. (2016)4. The CBS tools 3.0.2 (Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany) plugin for MIPAV 7.1.1 (Center for Information Technology, NIH, Bethesda, USA) was used for pre-processing (skull and dura mater stripping) the MP2RAGE volumes prior to the automatic segmentation by FreeSurfer using a modified version of the HCP high-res anatomical pipeline5. Subsequently, R1, T2*, cortical thickness and CBF were all projected onto the reconstructed cortical surface and coregistered to the common space (‘fsaverage’) to allow averaging across subjects and transformation to z-scores. These z-score maps were then used to map a subset of regions (based on the FreeSurfer Destrieux cortical atlas) based on the multiple features, with or without CBF, into a 2D space by means of dimensionality reduction for visualization of the level of similarity between regions.Results
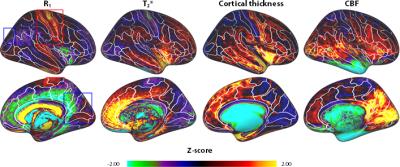
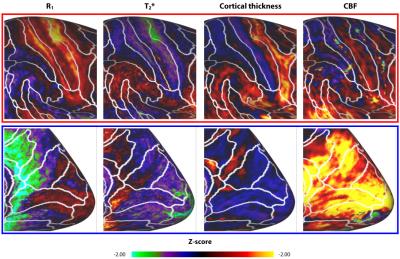
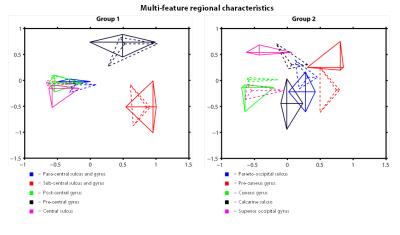
R1 and T2* cortical surface maps reveal a comparable pattern, as both are dominantly affected by the higher intra-cortical myelination of the primary areas (e.g. post-central gyrus, see red box in Figs. 1 and 2). Compared to these myelin-related maps, the cortical thickness map shows a different pattern, as e.g. illustrated by the difference in cortical thickness between the pre- and post-central gyri. The CBF data with smaller brain coverage (± 80%) exhibits a spatial pattern different from R1, T2* and cortical thickness, e.g. higher perfusion can be clearly observed in the occipital lobe and posterior cingulate, while R1 displays differences between these two areas. Fig. 3 illustrates the multi-feature-based differentiation among several regions (color coded) within the red (group 1) and blue (group 2) boxes of Fig. 2. The location on the 2D grid is determined by their shape (i.e. each spoke corresponds to a single feature) and visualizes the differences of MRI parameters for the various brain areas. This data clearly shows that the degree of the observed change, by including CBF information (solid vs. dashed glyphs), is different across groups of regions, i.e. group 1 vs. 2.Discussion
Here, we show the first application of multi-feature characterization of brain regions that includes CBF information, based on pre-defined regions. However, since CBF is closely coupled to brain metabolism6, a data-driven parcellation of brain regions that incorporates perfusion information will presumably enhance subsequent functional analysis. We can demonstrate that the CBF data is not redundant with the other quantitative MRI parameters. Therefore, we are currently setting up a fully automatic single-subject cortical parcellation routine based on hierarchical clustering that is driven by multiple parameters, which can be weighted differently to enhance clustering performance7,8.Acknowledgements
The authors are indebted to Prof. Dr. Andrew Webb (Leiden University Medical Centre, Leiden, Netherlands) and Dr. José Marques (Donders Institute for Brain, Cognition and Behaviour, Nijmegen, Netherlands) for providing the dielectric pads and the MATLAB code to perform the post-hoc T1 correction used in this study, respectively.References
[1] Glasser et al. Nature. 2016 Aug 11;536(7615). [2] Teeuwisse et al. Magn Reson Med. 2012 Apr;67(4). [3] Marques and Gruetter, PLoS One. 2013 Jul 16;8(7). [4] Ivanov et al. 2016 MRM 2016 doi: 10.1002/mrm.26351. [5] Glasser et al., Neuroimage. 2013 Oct 15;80. [6] Raichle M. PNAS. 1998 Feb 3; 95(3). [7] Pedregosa et al. JMLR. 2011 Nov 10;12. [8] Ward. JASA. 1963 Mar;58(301).
Figures