0347
Long-term Cerebrovascular Dysfunction Following Repeated Mild Traumatic Brain Injury1Sunnybrook Research Institute, Toronto, ON, Canada, 2Medical Biophysics, University of Toronto, Toronto, ON, Canada, 3Mouse Imaging Centre, The Hospital For Sick Children, Toronto, ON, Canada, 4Grenoble Institut des Neurosciences, Université Grenoble Alpes, Grenoble, France, 5Bruker Biospin MRI, Ettlingen, Germany, 6Inserm, U1216, Grenoble, France, 7Laboratory Medicine and Pathobiology, University of Toronto, Toronto, ON, Canada
Synopsis
Pseudo-Continuous Arterial Spin Labelling (pCASL) was used to assess cerebrovascular dysfunction of mice having traumatic brain injury (TBI) - which had been induced via serial controlled cortical impacts. Resting perfusion was quantified in absolute units via multiple post-label-delay pCASL experiments, and found to be reduced in the lesion. Furthermore, vascular reactivity to hypercapnic challenge, assessed via pCASL, appears to be enhanced in initial results. These results, in conjunction with immunohistochemical analysis and T2-weighted structural images, imply severe damage due to TBI, with vascular adaptation in the form of angiogenesis as the response from the brain.
Purpose
Traumatic Brain Injury (TBI) damages brain tissue, both directly and indirectly, by inducing ischemia and hypoxia.1 Rodent models of TBI have hitherto primarily employed single-impact TBI; in contrast, our model involves a series of impacts so as to more faithfully recapitulate the experience of some groups at high risk of suffering TBI - including athletes, and military personnel - and investigate possible synergistic effects of multiple impacts. The brain’s dependence on the continuous delivery of oxygen and other metabolites necessitates adaptation by the cerebral vasculature following injury.2 In particular, angiogenesis promoted by astrocytes, endothelial cells, neurons, and pericytes aims to restore healthy cerebral perfusion and mitigate ischemia. Such cerebrovascular adaptation motivates the investigation of cerebral blood flow (CBF) post TBI to elucidate the global sequelae of injury.3 In the present study we used Pseudo-Continuous Arterial Spin Labelling (pCASL) to quantify both resting perfusion and CBF reactivity to vascular perturbation in injured mice following repeated cortical insults.4Methods
TBI: Serial TBI preparation involved three impacts performed at four week intervals. Mice underwent craniotomy (2.5mm diameter) over the mid-parietal region, and cortical impacts were conducted to depth of 0.5mm below dural surface using a controlled cortical impactor (1.5mm diameter tip) travelling at 2m/s, and dwell time of 200μs.5
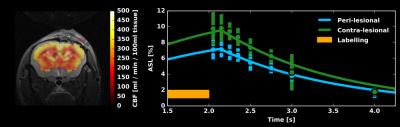
MRI: Structural and functional MRI, two weeks following the final impact, was performed using a 7T horizontal preclinical MRI system (Bruker Inc.). For all imaging, mice were anesthetized with isoflurane (5% induction, 1.5% maintenance). Temperature, heart rate, and breath rate were monitored throughout the imaging. T2 RARE data were collected with thirteen 0.7mm thick coronal slices, at 62.5μm nominal in-plane resolution, with TR/TE of 2500/33ms. For assessment of resting perfusion (n=4) and functional response to 10% CO2 (n=1), pCASL imaging was done with labelling using the unbalanced scheme (5μT pulses with Gav/Gmax=10/45mT/mm, Hanning shaped pulses of duration 0.4ms, 0.8ms pulse interval, with interpulse phase changes to optimize pCASL signal). Pulses were applied through a plane positioned perpendicular to the common carotid artery, 11mm posterior to the imaging slice. Single slice EPI images were then collected with TR/TE=3500/14ms, 1mm coronal slice thickness, FOV of 16mm x 16mm, and matrix size of 64x64. For multi-post label delay (PLD) experiments to quantify resting CBF, thirty labelled-unlabelled EPI pairs were acquired for each of eight PLDs (50ms, 150ms, 250ms, 350ms, 500ms, 750ms, 1000ms, and 2000ms). Resting CBF was computed in absolute units by modelling the pCASL signal vs. PLD using a single compartment model.6 For assessment of cerebrovascular reactivity to 10% CO2 in the inspired gas mixture, the PLD was set to 250ms and pCASL imaging was performed during one-minute hypercapnic challenges separated by four minutes of medical air breathing. Changes in pCASL signal were assessed via General Linear Model analysis performed with AFNI.
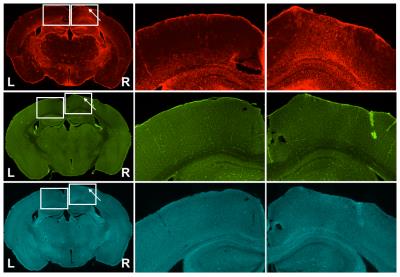
Immunohistochemistry: Immediately following imaging, brains were collected, sectioned via sliding freezing microtome (40µm) and stained for GFAP (astrocytic marker), Iba-1 (microglial and macrophage activation), and fluorescein-labelled lycopersicon esculentum (tomato) lectin (vascular endothelium). GFAP and Iba-1 sections were incubated with secondary antibody conjugated to AlexaFluor-594.
Results
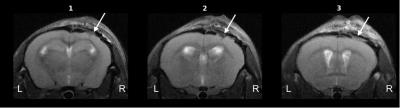
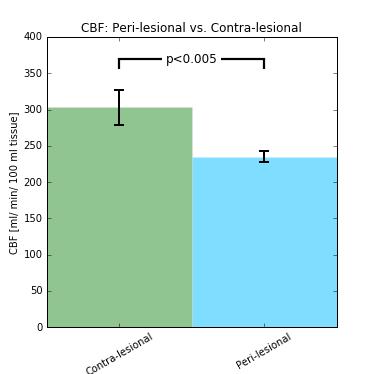
Structural images revealed severe tissue damage and cortical swelling at the site of the impact (Figure 1). pCASL was acquired in a slice containing structural damage. Multi-PLD experiments allowed absolute quantification of CBF both peri- and contra-lesionally (Figure 2). Peri-lesional CBF (235±8 ml/min/100ml tissue) was reduced relative to that of healthy cortical tissue (303±24 ml/min/100ml tissue); p<0.005 (Figure 3). Initial evidence suggests enhanced vascular reactivity in the lesion as the pCASL signal increased 13.01±0.02% from baseline in response to the 10% CO2 challenges, while the signal of the healthy tissue increased by 10.96±0.02%. Immunohistochemical analysis showed peri-lesional astrogliosis, consistent with reported inflammation following TBI (Figure 4). There were no significant changes in microglial recruitment and vessel density.Discussion
Following repeated TBI, we observed peri-lesional depression of resting CBF proximal to the lesion, suggesting an increase in vessel tone in the weeks following impact. Concomitantly, introducing a hypercapnic challenge appears to yield a greater increase in peri- (vs. contra-) lesional CBF. Reduced resting CBF with enhanced vascular reactivity could occur as a result of functional recruitment of newly added vessels (of greater resting tone) during vasodilatory challenges. Further immunohistochemical analysis at earlier time points and with a broad range of markers of vessel maturation is currently underway.Conclusion
In this study, the effects of TBI on cerebral blood flow was assessed via pseudo-continuous arterial spin labelling and histopathology. ASL imaging revealed reduced peri-lesional resting perfusion with enhanced cerebrovascular reactivity. Methods used here will be invaluable for monitoring the effects of TBI treatments targeting the cerebrovasculature.Acknowledgements
No acknowledgement found.References
1. Hayward NMEA, Tuunanen PI, Immonen R, Ndode-Ekane XE, Pitkänen A, Gröhn O. Magnetic resonance imaging of regional hemodynamic and cerebrovascular recovery after lateral fluid-percussion brain injury in rats. Journal of Cerebral Blood Flow & Metabolism. 2011;31(1):166-177.
2. Xing C, Hayakawa K, Lok J, Arai K, Lo EH. Injury and repair in the neurovascular unit. Neurological research. 2012;34(4):325-330.
3. Algattas H, Huang JH. Traumatic Brain Injury Pathophysiology and Treatments: Early, Intermediate, and Late Phases Post-Injury. International Journal of Molecular Sciences. 2014;15(1):309-341.
4. Hirschler L, Debacker CS, et. al. Robust Inter-Pulse Phase Correction for Brain Perfusion Imaging at Very High Field using Pseudo-Continuous Arterial Spin Labeling (pCASL). In Proceedings of the 23rd Annual Meeting of ISMRM, Toronto, Ontario, Canada. #3168
5. Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods 1991; 39; 253-262.
6. Parkes LM, Tofts PS. Improved Accuracy of Human Cerebral Blood Perfusion Measurements Using Arterial Spin Labeling: Accounting for Capillary Water Permeability. Magnetic Resonance in Medicine. 2002; (48):27-41.
Figures