0344
Golden Step, Golden Angle, Spiral-Cartesian Imaging for Flexible Gated Three-dimensional Angiography1Institute of Cardiovascular Science, University College London, London, United Kingdom, 2Centre for Medical Imaging, Division of Medicine, University College London, London, United Kingdom, 3Great Ormond Street Hospital for Children, London, United Kingdom
Synopsis
The purpose of this study was to develop and validate a novel 3D hybrid spiral-Cartesian MR angiographic sequence that utilized a golden step, golden angle acquisition strategy to enable flexible reconstruction.
We demonstrate that it is possible to acquire GSA-MRA and reconstruct it in a flexible manner (Real-time and cardiar and/or respiratory gated reconstructions). This may enable better interrogation of anatomy.
The new flexible 3D MRA provide novel ways of assessing cardiac disease.
INTRODUCTION
The purpose of this study was to develop and validate a novel 3D hybrid spiral-Cartesian MR angiographic (MRA) sequence that utilized a golden-step and golden-angle (GSA) acquisition strategy. Unlike conventional MRA this enables flexible reconstruction of both time resolved MRA, and cardiac gated MRA with and without respiratory self-navigation.METHODS
Patient population: 10 older children and adults with known cardiac disease.
Acquisition: The sequence was based on a 3D spiral-Cartesian (stack of spirals) acquisition(1). Cconsecutive spiral interleaves were first advanced by the golden-step in the kz direction with the same position in kx-ky (inner loop). Every 44 interleaves the position in kx-ky was rotated by the golden-angle (outer loop), Fig 1. Data was continuously acquired (during injection of contrast agent, Dotarem). The acquisitions parameters are presented in Tab.1.
Self-navigation: A respiratory navigator was calculated from a 1D projection of the imaged volume, calculated as the Fourier transform of 158 consecutive central k-space samples. Projections were updated in a sliding window fashion, with each new interleave. Signal changes due to cardiac motion and contrast passage were removed using two temporal frequency based adaptive filters (Fig. 1). The resulting self-navigator signal was compared to simultaneously acquired bellows data for validation.
Image reconstruction: To demonstrate the flexibility of the acquisition, GSA-MRA data were reconstructed as i) time resolved, ii) cardiac gated and iii) cardiac and respiratory angiograms. All reconstruction were performed off-line using a GPU based SENSE reconstruction(2). Vessel measurements and image quality metrics were compared to conventional breath-hold MR angiography (BH-MRA) in 10 patients.
A discrepancy metric (the maximum difference between the relative size of a sub-space and a relative number of points in it)(3) was used to assess the benefits of the GSA strategy on the uniformity of k-space filling. It was compared to two more commonly used strategies: the regular-angle, regular-step (RSA) and the regular-step, golden-angle (RSGA) trajectories (Fig. 2). Uniformity of filling was assessed for both cardiac/respiratory gated.
RESULTS
Time resolved MRA and cardiac gated MRA with and without respiratory self-navigation, were successfully reconstructed from GSA-MRA data in all 10 patients. The mean measured respiratory rate was 16.9±3.9 breaths per minute (12.1 to 22.9) and the mean heart rate was 66.5±10.1 beats per minute (53.7 to 81.0).
Fig. 3 shows imaging results in one patient from the cardiac gated, respiratory self-navigated GSA-MRA reconstruction. The respiratory self-navigation signal correlated well with the respiratory bellows; 0.78±0.06 (p<0.001). Respiratory gating was seen to improve vessel delineation and reduce motion artefacts (Fig. 4). Quantitatively edge sharpness (ES) was significantly lower in the free-breathing compared to the self-navigated cardiac gated GSA-MRA (0.48±0.26 / 0.92±0.33, p=0.014). Importantly, there was no significant difference in ES between the self-navigated GSA data and the same data reconstructed with navigator from the respiratory bellows (p=0.732).
Image quality metrics; Signal-to-Noise ratio, Contrast-to-Noise ratio and relative contrast, were significantly lower in the GSA-MRA reconstructions (p<0.001) than the BH-MRA acquisitions. However, signal homogeneity in the vessel was similar across all reconstructions (p>0.05).
Vessel diameter measurements performed at three aortic positions (ascending, isthmus, diaphragm) and three pulmonary artery positions (main; MPA, right; RPA, and left; LPA) showed excellent agreement between BH-MRA (22.1±6.7, 12 to 45.2mm) and the cardiac gated, self-navigation GSA-MRA reconstruction at peak diastole (21.8±6.6, 11.8 to 44.8mm, p=0.055), with significantly higher peak systolic measurements in all segments (24.0±6.9, 13.1 to 47.6mm, p<0.001).
For the cardiac and respiratory gated reconstructions there was no difference in the discrepancy between RSA (0.145±0.038) and RSGA (0.134±0.027) trajectories (p=0.455). However, the GSA (0.100±0.020) had significantly lower discrepancy (RSA p<0.001, RSGA p=0.004).
DISCUSSION
We implemented a novel MRA acquisition scheme based on GSA acquisition, that could be reconstructed both in a time resolved and cardio-respiratory gated fashion. We demonstrated that it is possible to perform robust respiratory navigation using a signal generated from the data itself.
Image quality assessments showed ES gains from cardiac gating were lost due to respiratory motion. However, the addition of the respiratory navigation significantly improved ES. The self-navigation signal was shown to have a good correlation with simultaneously acquire bellows data.
We showed good correlation between BH-MRA vessel measurements and GSA-MRA measurement in both systole and diastole.
The GSA showed better k-space filling (lower discrepancy) compared to more conventional acquisition schemes and enables retrospectively adjustable temporal resolution. Further, due to incoherent undersampling, it is compatible with compressed sensing reconstruction, which is planned in the future.
CONCLUSION
The GSA strategy allows flexible reconstruction of angiographic data, which may provide novel ways of assessing cardiac disease.Acknowledgements
No acknowledgement found.References
1. Steeden JA, Pandya B, Tann O, Muthurangu V. Free breathing contrast-enhanced time-resolved magnetic resonance angiography in pediatric and adult congenital heart disease. J Cardiovasc Magn Reson 2015;17:38.
2. Kowalik GT, Steeden JA, Pandya B, et al. Real-time flow with fast GPU reconstruction for continuous assessment of cardiac output. J Magn Reson Imaging 2012;36(6):1477-1482.
3. Drmota M, Tichy RF. Sequences, discrepancies, and applications. Berlin ; New York: Springer; 1997; xiii, 503 p. p.
Figures
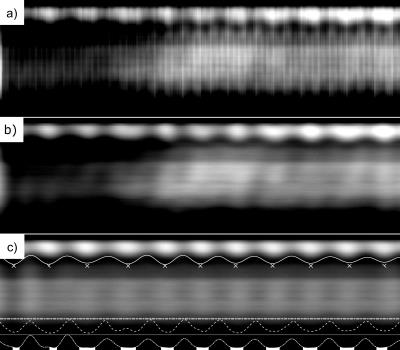
Fig. 1. The respiration navigator.
a) The raw cardiac and blood contrast corrupted signal. b) Cardiac free signal was achieved using adaptive low-pass filtering. c) The final signal was achieved using a band-pass filter around the highest peak in the respiration range.
The final result was used to extract the centre-of-mass variations (the solid line). The peak-expiratory projections (marked with x) were averaged to create a reference projection. This was correlated with the rest of data (the ‘-.’ line) to select 30% most expiratory read-outs (the white area selection). For comparison, the above it ‘--‘line represents the bellows signal.
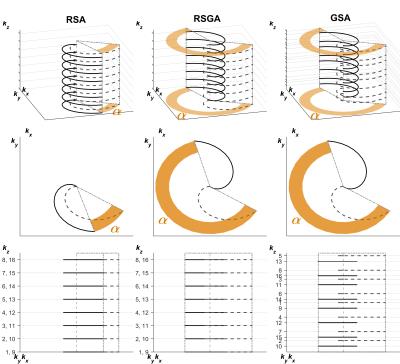
Fig. 2. Representation of the acquisition strategy of the golden-step, golden-angle (GSA), the regular-angle, regular-step (RSA) and the regular-step, golden-angle (RSGA) trajectories.
The strategy reduces the 3D k-space (top row; kx,ky,kz) to equivalent 2D description of changes ( ,kz). The inner-loop advances the read-out partition’s position (bottom row; shifts in kz by ~0.618 for GSA and 1/number-of-partitions for RSA, RSGA) and the outer-loop changes the rotation angle (middle row; angular shift of ~222o for GSA, RSGA and 1/number-of-interleaves for RSA). Solid line – current, dashed line – next outer-loop iteration. The interleave indices (bottom row) shows distribution of read-outs in kz.
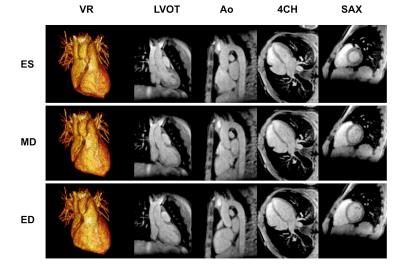
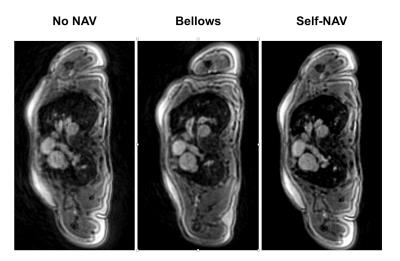
Fig. 4. Example of a multi-planar reformatted through the aortic root for the three cardiac gated reconstructions.
Note that the non-respiratory gated reconstruction (No NAV) exhibits breathing artefacts and vessel edge blurring. These were not present in either the bellows respiratory-gated (Bellows) or self-respiratory gated (Self-NAV) reconstructions.