0336
Ultra-High Spatiotemporal Resolution 4D Flow for Valve and Coronary Arterial Delineation1Department of Radiology, Stanford University, Stanford, CA, United States
Synopsis
4D flow provides single sequence rapid protocol for assessment of blood flow, ventricular function, and anatomy in congenital heart disease. Though valve function has been well validated for regurgitant flow fraction, assessment of valve leaflet delineation has been limited by relatively low spatiotemporal resolution. Similarly, coronary origin delineation has been limited. Here, we develop an ultra-high spatiotemporal resolution 4D flow acquisition technique and assess its performance for valve and coronary delineation. 4D flow provides superior delineation of coronaries than echo, is highly likely to depict coronary origins, and is highly likely to provide good valve leaflet delineation.
Purpose
Four-dimensional (4D) flow1–3 provides single-sequence rapid protocol for assessment of blood flow, ventricular function, and anatomy in congenital heart disease4. Though valve function has been well validated for regurgitant flow fraction5, assessment of valve leaflet motion and delineation has been limited by relatively low spatiotemporal resolution. Similarly, coronary origin delineation has been limited. Here, we develop an ultra-high spatiotemporal resolution 4D flow acquisition technique and assess its performance for valve and coronary delineation.Material and Methods
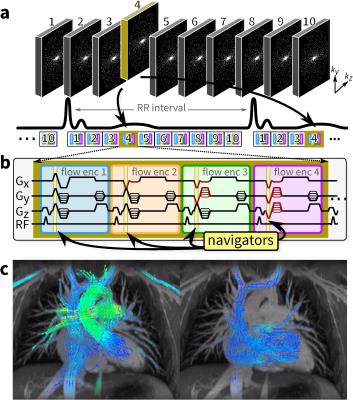
An RF-spoiled GRE sequence with four-point velocity encoding6 was modified to enable 3D Cartesian variable-density pseudo-random sampling design7, illustrated in Figure 1a to 10 phases, but which can be prescribed and reconstructed to the desired temporal resolution. With four velocity encodings repeated throughout the scan, velocity-encoding gradients are leveraged to provide intrinsic navigation with no scan time penalty7 (Figure 1b). Datasets are reconstructed using auto-calibrated parallel imaging (with ESPIRiT8 and compressed sensing (with spatial wavelet sparsity and temporal sparsity9,10) along with respiratory soft-gating7,11. All reconstructions were performed using BART12.
Twenty consecutive pediatric patients (0.5–19 years, 14 males and 6 females, heart rate of 90±16 bpm) referred for 3T cardiac MRI were recruited. Post-ferumoxtyol 4D flow MRI was obtained with a 32-channel cardiac coil, an acquisition matrix of 224x224, and a field of view tailored to cover the chest. Slice thickness was 1.4 mm for 120 slices to enable whole chest coverage and velocity encoding was 250 cm/s. Datasets were acquired with ≥30 true frames per cardiac cycle which corresponds to a temporal resolution of 34.5±7.7 ms. With a reduction factor of 15 before k-space corner cutting, scan times were 10–15 min.
Two cardiovascular radiologists independently scored each valve on a three-point scale for leaflet delineation, motion depiction, and coaptation. Presence of regurgitation and stenosis were recorded. Coronary origin delineation was also scored on a three-point scale. For each patient, the report for the closest echo without intervening catheter or surgical intervention was reviewed to determine presence of valvular stenosis or regurgitation and delineation of coronary origins. Mean scores were computed, along with confidence interval of not obtaining a highest quality score. Proportion of agreement between echo and MRI for valvular dysfunction was assessed.
Results
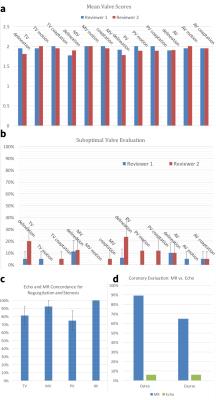
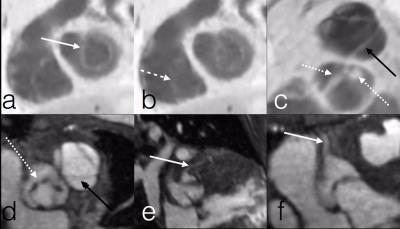
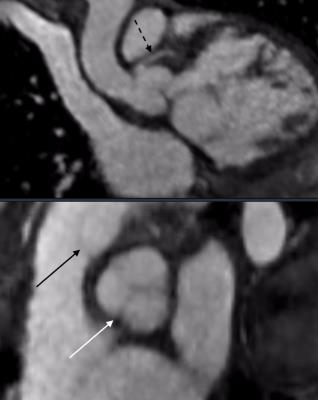
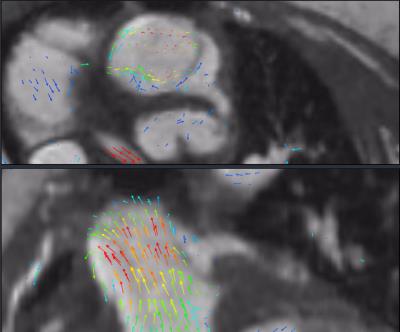
The mean scores of valve image quality are shown in Figure 2a. Additionally, the percentage of suboptimal images, defined as a score of less than 2, is plotted in Figure 2b. These data confirm the adequate assessment of valve morphology, motion, and coaptation. 80% confidence intervals of obtaining suboptimal assessment of valves were under 20% for all features, except rose to 30% for tricuspid valve delineation and 40% for pulmonary valve delineation. Agreement between echo and MRI for valvular pathology was moderate (Figure 2c). The coronary arteries were seen significantly better with MRI than with echo, including not only location of ostium but also proximal course (Figure 2d). Representative examples in Figures 3-5 demonstrate good valvular leaflet and coronary artery delineation.Discussion
High spatiotemporal resolution enabled by the proposed approach enables high likelihood of visualizing small pediatric coronary arteries prone to rapid motion. The rapid movement of valves can be similarly resolved and visualized. There were some discrepancies of valvular regurgitation and stenosis between MRI and echo: this may, in part, stem from months passing between the two studies and from different physiologic states for exams performed with sedation. Although ventricular wall motion was not specifically evaluated and usually does not have focal abnormalities in congenital heart diseases, the temporal resolution afforded by 4D flow in this study exceeds that of typical adult bright blood imaging.
At higher temporal resolutions, neighboring cardiac phases become more and more similar which result in sparser representation of the dataset along the cardiac-motion dimension. Thus, with high SNR from contrast-enhancement, we hypothesize that higher acceleration factors may be possible to further reduce scan durations. Also, at these high temporal resolutions, accuracy of determining cardiac trigger points become especially important.
Conclusion
Valve morphology, motion, and coaptation can be well depicted by incorporating variable-density pseudo-randomly-ordered Cartesian k-space acquisition and intrinsic navigation. Coronary artery origins are likely to be well delineated. This further expands the comprehensive nature of the assessment of congenital heart diseases afforded in a single-sequence protocol of ferumoxytol-enhanced 4D flow.Acknowledgements
NIH R01-EB009690, NIH R01-EB019241, NIH P41-EB015891, Tashia and John Morgridge Faculty Scholars Fund, and GE Healthcare.References
1. Kozerke S., Hasenkam JM., Pedersen EM., Boesiger P. Visualization of flow patterns distal to aortic valve prostheses in humans using a fast approach for cine 3D velocity mapping. J Magn Reson imaging 2001;13:690–698.
2. Wigström L., Sjöqvist L., Wranne B. Temporally resolved 3D phase-contrast imaging. Magn Reson Med 1996;36:800–803.
3. Markl M., Chan FP., Alley MT., Wedding KL., Draney MT., Elkins CJ., et al. Time-resolved three-dimensional phase-contrast MRI. J Magn Reson Imaging 2003;17:499–506.
4. Vasanawala SS., Hanneman K., Alley MT., Hsiao A. Congenital heart disease assessment with 4D flow MRI. J Magn Reson Imaging 2015;42:870–886.
5. Hsiao A., Lustig M., Alley MT., Murphy MJ., Vasanawala SS. Evaluation of Valvular Insufficiency and Shunts with Parallel-imaging Compressed-sensing 4D Phase-contrast MR Imaging with Stereoscopic 3D Velocity-fusion Volume-rendered Visualization. Radiology 2012;265:87–95.
6. Pelc NJ., Bernstein MA., Shimakawa A., Glover GH. Encoding strategies for three-direction phase-contrast MR imaging of flow. J Magn Reson imaging 1991;1:405–413.
7. Cheng JY., Hanneman K., Zhang T., Alley MT., Lai P., Tamir JI., et al. Comprehensive motion-compensated highly accelerated 4D flow MRI with ferumoxytol enhancement for pediatric congenital heart disease. J Magn Reson Imaging 2016;43:1355–1368.
8. Uecker M., Lai P., Murphy MJ., Virtue P., Elad M., Pauly JM., et al. ESPIRiT-an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn Reson Med 2014;71:990–1001.
9. Lustig M., Donoho D., Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med 2007;58:1182–1195.
10. Feng L., Srichai MB., Lim RP., Harrison A., King W., Adluru G., et al. Highly accelerated real-time cardiac cine MRI using k - t SPARSE-SENSE. Magn Reson Med 2013;70:64–74.
11. Johnson KM., Block WF., Reeder SB., Samsonov A. Improved least squares MR image reconstruction using estimates of k-Space data consistency. Magn Reson Med 2012;67:1600–1608.
12. Uecker M., Ong F., Tamir JI., Bahri D., Virtue P., Cheng JY., et al. Berkeley Advanced Reconstruction Toolbox. In: Proceedings of the 23rd Annual Meeting of ISMRM, Toronto, Ontario, Canada, 2015. (abstract 2486).
Figures