0334
A Preliminary Report on Time-Resolved Coronary Vessel Wall MRI in Heart Transplant Recipients1Department of Radiology, University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 2Division of Imaging Sciences and Biomedical Engineering, King’s College London, London, United Kingdom, 3Centre for Biomedical Imaging (CIBM), Lausanne, Switzerland, 4Department of Radiology, University Hospital (CHUV) of Lausanne, Lausanne, Switzerland, 5Service de Cardiologie, University Hospital (CHUV) of Lausanne, Lausanne, Switzerland
Synopsis
In this study, we applied a recently developed and robust coronary vessel wall MRI method that includes a golden angle trajectory and k-t sparse SENSE to a cohort of heart transplant patients. The coronary vessel wall was visualized with high contrast in all patients and the method holds great promise for quantitative and non-invasive characterization of coronary allograft vasculopathy (CAV) in transplanted patients.
Introduction
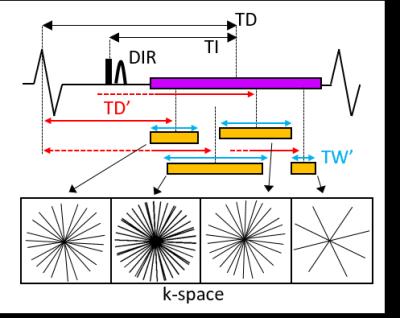
Cardiac Allograft Vasculopathy (CAV) is one of the complications after heart transplantation that may contribute to allograft rejection. Currently, CAV can be detected with invasive angiography which, however, may not be able to detect early stages of CAV that include remodeling of the vessel wall without luminal narrowing. Invasive intravascular ultrasound (IVUS) or optical coherence tomography (OCT) provide powerful alternatives, yet OCT suffers from limited penetration depth and cannot visualize the full extent of the coronary vessel wall. On this background, coronary vessel wall MRI1 may be a valuable alternative2. While the spatial resolution of MRI is inferior to that of IVUS or OCT, it is non-invasive and not limited to the visualization of the inner layers of the coronary vessel wall. Typically, coronary vessel wall MRI is obtained using double inversion recovery (DIR) that suppresses the signal from the coronary lumen, which leads to the visualization of the coronary vessel wall with high contrast. DIR acquisitions are difficult to plan as optimal blood signal suppression3 and cardiac motion compensation have to be simultaneously achieved. Recently, a fully flexible, time resolved MRI acquisition and reconstruction framework4 based on the golden angle trajectory5 and k-t-sparse SENSE6 has been introduced. This technique includes a continuous acquisition throughout a prolonged acquisition window in conjunction with a posteriori selection of optimal reconstruction parameters (Fig1). This added flexibility significantly improved the image quality and success rate in healthy volunteers when compared to conventional DIR4. In this study, we applied this method for the first time in a patient cohort of heart transplant recipients who may ultimately benefit from a non-invasive and quantitative characterization of the coronary vessel wall.Methods
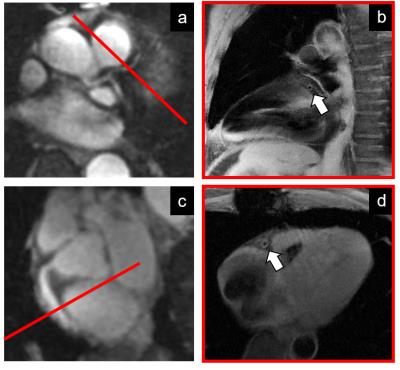
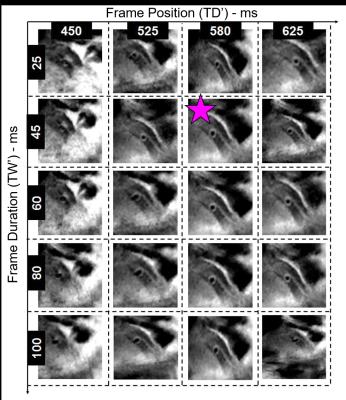
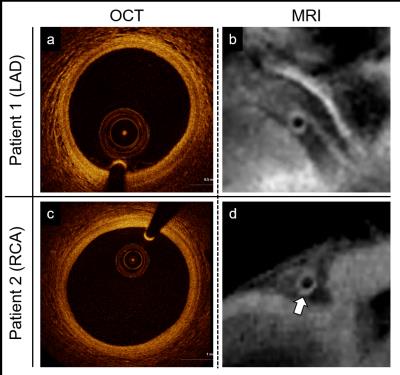
Data acquisition was performed on a 3T system (Siemens MAGNETOM Prisma) using a 2D radial DIR golden angle gradient echo (GRE) imaging sequence. Images were acquired in N=7 heart transplant recipients (4 males, 51.6±13.6years) and all the acquisitions were performed after injection of Gadovist (0.1ml/kg). A 3D whole-heart dataset was acquired prior to the DIR acquisition for slice localization perpendicular to the right (RCA) or the left anterior descending (LAD) coronary artery (Fig2). Imaging parameters for the 2D coronary vessel wall GRE acquisition included: radiofrequency (RF) excitation angle=16°, FOV=(260mm)2, TE=3.2ms, TR=6.5ms, slice thickness=8mm, in-plane resolution=(0.8mm)2, TI=300-350ms. For each individual case, 50-80 frames were reconstructed in Matlab, with a temporal duration ranging from 25 to 100ms (TW’, Fig1) and for different time points in the cardiac cycle (TD’, Fig1). For each patient, the frame showing the best visual vessel wall conspicuity was selected and used for data analysis (Fig3). For each of these frames, visible vessel wall circumference (VWC), vessel wall sharpness (%VWS), and vessel wall thickness (VWT) were computed7. Simultaneously, the related a posteriori reconstruction parameters, consisting of optimized trigger delay (TD*) and optimized temporal width of the reconstructed frames (TW*) were recorded. Furthermore, and in two patients (Fig4), OCT data were also available. For these, intima (I) and media (M) thickness, as well as I/M ratio were quantified.Results
Data acquisition and reconstruction was successfully completed in all cases. Adequate vessel wall delineation was obtained in 7 out of 7 patients (100% success rate). Quantified endpoints included 9.2±4.5mm, 33.2±9.5%, and 1.69±0.35mm for VWC, %VWS, and VWT, respectively. On average, an optimized trigger delay TD* of 490.8±103.7ms was selected, with a temporal frame width TW* of 58.0±18.0ms. From OCT images, I=100µm and M=160µm (I/M ratio=0.62) was measured for Patient 1 (MRI-derived VWT=1.60mm) and I=210µm and M=140µm (I/M ratio=1.5) for Patient 2 (MRI-derived VWT=1.77mm) (Fig4).Discussion and Conclusions
In this preliminary study, a time-resolved framework for coronary vessel wall DIR was successfully tested (100% success rate) in a cohort of heart transplant recipients, which suggests that coronary vessel wall MRI is feasible in a challenging clinical setting. As reported in the Results, VWT was 1.69±0.35mm in our studied cohort of patients with transplanted hearts. To put this into perspective, in a previous study in healthy adult subjects, we have measured an average VWT of 0.97±0.18mm using the same methodology albeit without the use of contrast injection4. This advances the hypothesis that VWT is increased in our study cohort but it remains to be elucidated whether the contrast agent, age, vasculopathy or a combination thereof are contributing factors. Preliminary individual comparisons between OCT and MRI showed to be in good agreement (Fig4); therefore, a more extensive gold standard comparison in which MRI measures are quantitatively compared to those from IVUS or OCT is now warranted.Acknowledgements
This work has been supported by the Swiss National Science Foundation Grants 320030_143923 and 326030_150828 and the Centre d’Imagerie BioMedicale (CIBM).References
1) Kim WY, et al. Three-dimensional black-blood cardiac magnetic resonance coronary vessel wall imaging detects positive arterial remodeling in patients with nonsignificant coronary artery disease. Circulation 2002; 106:296-299.
2) Hussain T, et al. Detection and grading of coronary allograft vasculopathy in children with contrast-enhanced magnetic resonance imaging of the coronary vessel wall; Circulation: Cardiovascular Imaging. 2013;6:91-98.
3) Fleckenstein JL, et al. Fast short-tau inversion recovery MR imaging. Radiology 1991; 179:499-504.
4) Ginami G, et al. Golden angle dual-inversion recovery acquisition coupled with flexible time-resolved sparse reconstruction facilitates sequence timing in high-resolution coronary vessel wall MRI at 3T. Magn Reson Med 2016; DOI 10.1002/mrm.26171.
5) Winkelmann S, et al. An optimal radial profile order based on the golden ratio for time-resolved MRI. IEEE Trans Med Imaging 2007; 26(1):68-76.
6) Feng L, et al. Highly accelerated real-time cardiac cine MRI using k-t sparse SENSE. Magn Reson Med 2013; 70(1):64-74.
7) Etienne A, et al. “Soap-Bubble”: Visualization and quantitative analysis of 3D coronary magnetic resonance angiograms. Magn Reson Med 2002; 48:658-66.
8) Khandhar SJ, et al. Optical coherence tomography for characterization of cardiac allograft vasculopathy after heart transplantation (OCTCAV study). J Heart Lung Transplant 2013; 32(6):596-602.
Figures