0333
Whole-Brain Vessel Wall MR Imaging Using Inversion-Recovery Prepared SPACE: Reproducibility and Accuracy of Intracranial Artery Morphology1Biomedical Imaging Research Institute, Cedars Sinai Medical Center, Los Angeles, CA, United States, 2Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, People's Republic of China, 3Siemens Healthcare
Synopsis
Intracranial atherosclerosis disease is one of the main causes for cerebrovascular events. A T1-weighted whole-brain vessel wall imaging method, inversion-recovery prepared SPACE (IR-SPACE), was developed at 3T demonstrating advantages over conventional SPACE. This study was aimed to determine the reproducibility of scan-rescan, intra-, and inter-observer as well as the accuracy when using the technique for morphology assessment of the intracranial vessel wall. In general, this study demonstrated excellent reproducibility and good agreement between the 3D and 2D techniques. In conclusion, IR-SPACE is a reproducible and accurate MR method for intracranial vessel wall imaging.
Introduction
Intracranial atherosclerosis disease (ICAD) is one of the main causes for cerebrovascular events. Studies have demonstrated that high resolution black-blood MR, particularly using 3D turbo spin-echo (TSE) with variable refocusing flip angles (SPACE), is an effective technique for assessing ICAD [1,2]. Recently, a T1-weighted whole-brain vessel wall imaging method, inversion-recovery prepared SPACE (IR-SPACE), was developed at 3T demonstrating advantages over conventional SPACE in T1 contrast weighting, signal suppression of cerebrospinal fluid, and spatial coverage [3,4]. To better suit clinical needs, such an imaging method ought to be accurate and reproducible. This study was aimed to determine the reproducibility of scan-rescan, intra-, and inter-observer as well as the accuracy when using the technique for morphology assessment of the intracranial vessel wall.Materials and Methods
This study was IRB approved and the written informed consent obtained from all subjects before scanning. Thirty-four healthy volunteers (24 M; age 31- 66 yrs; mean age 49.74 yrs) underwent two repeated IR-SPACE scans on a 3T MR system equipped with a 32-channel head coil. Relevant imaging parameters were: spatial resolution 0.53×0.53×0.53 mm3, sagittal orientation, GRAPPA 2, scan time 7-8 min [4]. Subjects were given a break by getting off the scanner table before the second scan. In 19 out of the 34 subjects, T1-weighted 2D TSE (spatial resolution 0.53×0.53×2 mm3) was also performed at 3 contiguous cross-sections of 3 selected arterial segments reformatted from IR-SPACE images. The 2D TSE images served as an MR imaging reference for intracranial artery morphology. Lumen and vessel wall volume, normalized wall index, mean and maximum wall thickness were measured from both IR-SPACE and corresponding 2D TSE images at matched locations and slice thickness. Five vessel segments over a 6-mm length were analyzed, including the distal basilar artery (BA), the distal vertebral artery (V4), the distal internal carotid artery supaclinoid segment (C4), and the proximal middle cerebral artery (MCA) M1 and the proximal anterior cerebral artery (ACA) A1. Using commercial software (VesselMass, Leiden University Medical Center), two readers (with 6-year and more than 10-year experience in vascular MR, respectively) independently performed above geometric measurements on the images from the first IR-SPACE scan. After two weeks, one reader performed a second-round measurement on the same data, and the other performed measurement on the images from the second scan as well as 2D TSE scan. Intra- and inter-observer agreement and scan-rescan reproducibility of IR-SPACE as well as the agreement between IR-SPACE and 2D TSE were calculated.Results
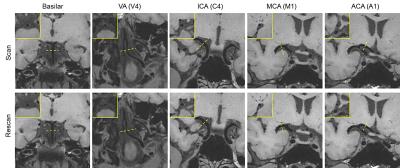
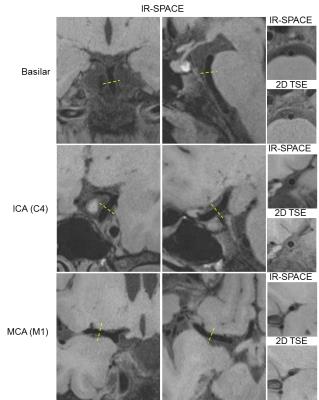
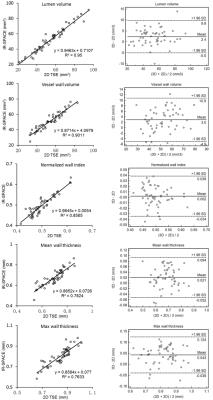
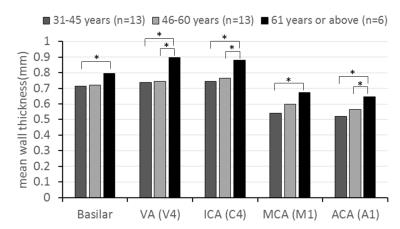
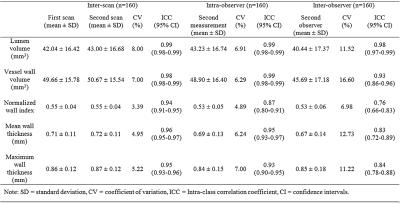
All subjects successfully underwent scans. Two subjects were excluded from analysis due to motion-induced poor image quality. The IR-SPACE sequence provided good or excellent delineation of vessel wall and large spatial coverage (Fig. 1). All morphologic measurements and corresponding coefficient of variation (CV), intra-class correlation coefficients (ICC) and 95% confidence interval were summarized in Table 1. All measurements demonstrated excellent reproducibility. For scan-rescan reproducibility, all ICC were larger than 0.9 with CV ranging from 3.4% to 8.0%. Except for inter-observer reproducibility on normalized wall index (ICC=0.76), all ICC were larger than 0.8 with CV ranging from 4.9% to 7.0% and 7.0% to 16.6% for intra- and inter-observer reproducibility, respectively,. IR-SPACE and 2D TSE provided visually comparable vessel wall delineation(Fig. 2). The linear regression analysis and Bland-Altman plots revealed good agreement between IR-SPACE and 2D TSE (Fig. 3). Strong correlation (r ≥ 0.95) was observed for lumen and vessel wall volume. In addition, mean wall thickness was grouped by age (Fig. 4). Significant difference was observed for each of assessed arterial segments between age group of 31-45 years and group of 61 years or above and for VA, ICA, and ACA between group of 46-60 years and group of 61 years or above.Discussion and Conclusion
In general, this study demonstrated excellent reproducibility of scan-rescan, intra-, and inter-observer. Both ICC and CV obtained here are in good agreement with those reported in previous studies on either intracranial or carotid arteries [5-8]. With such high reproducibility, IR-SPACE can potentially be used for monitoring therapy and disease progression and, more importantly, fewer participants will be required for a therapeutic trial enrollment. Good agreement between the 3D and 2D techniques suggests that IR-SPACE is an accurate imaging approach to morphologic assessment of intracranial vessel wall. Using this method, we demonstrated that aging from 31-45 years to 61 years or above is associated with an increase of 0.08-0.16 mm in mean wall thickness. In conclusion, IR-SPACE is a reproducible and accurate MR method for intracranial vessel wall imaging and potentially useful for ICAD assessment.Acknowledgements
This work was supported in part by American Heart Association (15SDG25710441), National Institutes of Health (NHLBI 2R01HL096119).References
[1] Qiao Y et al. JMRI 2011; 34:22-30;
[2] van der Kolk AG et al. Eur Radiol. 2013; 23:2996-3004.
[3] Fan Z et al. MRM 2016; [Epub ahead of print].
[4] Fan Z et al. ISMRM 2016;4378.
[5] Qiao Y, et al. Radiology 2016;280:860-868.
[6] Li F et al. J Magn Reson Imaging 2010;31:168-176.
[7] Alizadeh Dehnavi R, et al. J Magn Reson Imaging 2007;25:1035-1043.
[8] Kröner ES, et al. Eur J Radiol 2013;82:680-685.
Figures