0328
Hyperpolarized Micro-NMR for Metabolic Flux Analysis in Cancer Stem Cells and Rapid Assessment of Therapeutic Response1Department of Radiology/Molecular Pharmacology Program, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Center for Cell Engineering/Molecular Pharmacology Program, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 3Center for Systems Biology, Massachusetts General Hospital, Boston, MA, United States, 4Department of Radiology, Harvard Medical School, Boston, MA, United States, 5Weill Cornell Medical College, New York, NY, United States
Synopsis
Aberrant metabolic features of cancer cells are closely related to tumorigenesis and therapeutic response. Here, we report a sensitive magnetic resonance sensing platform, capable of analyzing metabolic fluxes in mass-limited samples. Termed hyperpolarized micromagnetic resonance spectrometer (HMRS), this platform achieved to characterize the metabolic flux in cancer stem cells in real-time and assess therapeutic responses much earlier than any changes in cell viability. This will become a versatile platform for rapid and sensitive exploration of metabolic dynamics in cancer.
INTRODUCTION
Aberrant metabolic reprogramming is a unique feature of cancer cells, closely related to multiple oncogenic signaling pathways.1 The metabolic rate or ‘flux’ can represent the metabolic activity of cellular biochemical reactions, and its analysis can describe how cancer cells respond to drug treatment, which may reveal novel therapeutic targets in cancer. Particularly, a comprehensive understanding of metabolic flux in cancer stem cells is necessary to eradicate cancers as these cells are resistant to various therapies and cause relapse.2 Mass spectrometry and optical methods have been used to study cancer metabolism, but they are destructive and require extended periods of incubation with labeled molecules, which could distort metabolic reactions. There is a great need for sensitive approaches to assess metabolic flux non-destructively and rapidly in mass-limited samples, such as cancer stem cells. Here, we report a novel analytical platform, termed hyperpolarized micromagnetic resonance spectrometer (HMRS), capable of probing endogenous metabolism in 104 live cells using hyperpolarized probes. We validated this system in multiple cell lines and extended it to access drug treatment response and characterize cancer stem cells in vitro.METHODS
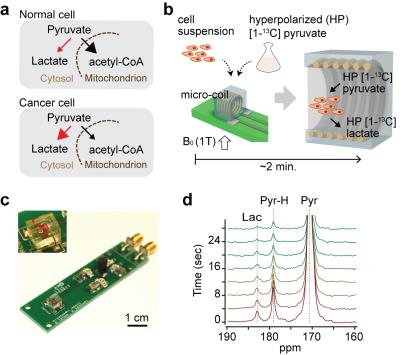
The HMRS platform exploits (i) hyperpolarization
of metabolites and (ii) miniaturization of a detection coil. Hyperpolarization,
which can radically increase NMR signal (>10,000 fold), allows real-time
monitoring of metabolism.3 We used [1-13C] pyruvate as a hyperpolarized
probe because its spin-relaxation time (T1) is relatively long (~70 sec
at 1 Tesla)4 and its metabolic reaction to lactate is normally
elevated in cancer cells5 (Fig. 1a).
The hyperpolarized pyruvate was mixed with cell suspension and loaded into a
miniaturized NMR coil in the HMRS system for metabolic flux analyses (Fig. 1b). The miniaturized coil (1.4-mm
diameter, 2 μL),
which was implemented inside a polydimethylsiloxane (PDMS) block, increased the filling-factor of the target molecules and enhanced the
detection sensitivity (>100-fold compared to a conventional NMR coil) (Fig. 1c). The HMRS system acquired NMR spectra every four seconds
with a 30° RF pulse and quantified them based on the peak area (Fig. 1d). For cancer stem cell study,
we used MLL-AF9 acute myeloid leukemia (AML) mouse model. For drug treatment
study, we used K562 chronic myeloid leukemia (CML) cell line.
RESULTS and DISCUSSION
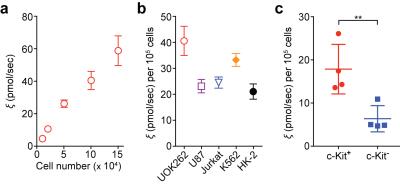
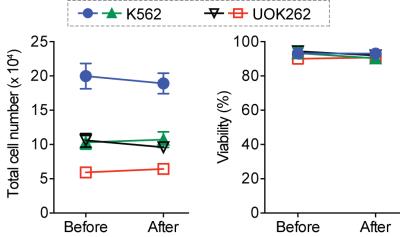
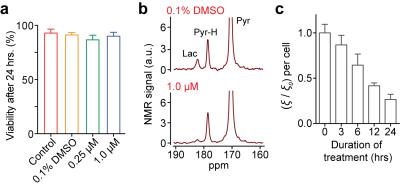
The HMRS platform achieved the metabolic flux analysis in cancer cells as low as 104 cells, with a linear response to cell numbers (R2 > 99%) (Fig. 2a). It is important to note that conventional hyperpolarized NMR techniques require larger number of cells (> 107), more than 3 orders of magnitude greater than this platform. We then profiled five different cell lines reproducibly (Fig. 2b). As expected, quantified conversion of hyperpolarized pyruvate to lactate was identical to previous studies,6 though here using 103 fold less cells. Next, we applied the HMRS platform to investigate the metabolic flux in primary leukemia stem cells, where the number of cells is limited and using conventional hyperpolarized NMR technique is impractical. Intriguingly, the leukemia stem cells (c-Kit+) demonstrated nearly two-fold higher flux than leukemia non-stem cells (c-Kit-) (Fig. 2c). This metabolic feature could be explained by the differential expression level of Myc, which regulates metabolite transporters as well as lactate dehydrogenase A (LDHA).7,8 The HMRS platform has another advantage: samples remain intact and can be retrieved after experiments. The signal detection was based on magnetic resonance, and the miniaturized coil circuit was implemented inside a hydrophobic polymer block. The cell loss was negligible and their viability was not changed significantly during the experiments (Fig. 3). This reaffirmed that mass-limited samples could be analysed by the HMRS in tandem with other techniques, providing a method directly connecting metabolism to other molecular characteristics. We also applied the HMRS platform for monitoring drug-treatment efficacy. Since metabolic changes in cells are prerequisite for any clinicopathological changes, metabolic flux analyses can provide rapid assessment of treatment efficacy. Using a tyrosine kinase inhibitor with chronic myeloid leukemia as a model, the HMRS enabled to detect the drug effect as early as 3 hours of post-treatment, well before any change in viability of the cancer cells was detected (Fig. 4).CONCLUSION
The HMRS platform, exploiting the advantages of hyperpolarized NMR and the miniaturized detection coil, enabled non-destructive and rapid analyses of metabolic flux in a small number of cells. It succeeded to profile metabolic fluxes in cancer stem cells and assess therapeutic responses in cancer cells much earlier than any changes in viability. With further optimizations, the HMRS will become a more versatile platform for rapid and sensitive exploration of metabolic dynamics in biologically relevant systems.Acknowledgements
The authors thank Dr. Sui Seng Tee and Dr. Valentina Di Gialleonardo for helping the western blot analysis and providing insightful comments on experimental details. This work was supported in part by US NIH grants R01HL113156 (H.L.), R21CA205322 (H.L.), R00EB014328 (K.R.K.), Cancer Center Support Grant P30CA008748 (K.R.K.), Department of Defense Ovarian Cancer Research Program Award W81XWH-14-1-0279 (H.L.) as well as the Center for Molecular Imaging and Nanotechnology (CMINT) at MSKCC.References
1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674.
2. Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17(3):313-319.
3. Ardenkjaer-Larsen JH, Fridlund B, Gram A, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci USA. 2003;100(18):10158-10163.
4. Tee SS, DiGialleonardo V, Eskandari R, et al. Sampling Hyperpolarized Molecules Utilizing a 1 Tesla Permanent Magnetic Field. Sci Rep. 2016;6(August):32846.
5. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029-1033.
6. Keshari KR, Sriram R, Koelsch BL, et al. Hyperpolarized 13C-pyruvate magnetic resonance reveals rapid lactate export in metastatic renal cell carcinomas. Cancer Res. 2013;73(2):529-538.
7. Stine ZE, Walton ZE, Altman BJ, Hsieh AL, Dang C V. MYC, metabolism, and cancer. Cancer Discov. 2015;5(10):1024-1039.
8. Park SM, Gönen M, Vu L, et al. Musashi2 sustains the mixed-lineage leukemia-driven stem cell regulatory program. J Clin Invest. 2015;125(3):1286-1298.
Figures