0320
Early screening of pancreatic iron overload in thalassemia major with MRI T2*1Sun Yat-sen Memorial Hospital, Guangzhou, People's Republic of China, 2Guangdong Medical College, People's Republic of China
Synopsis
Diabetes Mellitus is a serious complication of thalassemia major. Intensive chelation therapy in the early stage may avoid diabetes. So we aimed to determine the optimal timing age of pancreatic iron screening with MRI T2* technique. Early pancreatic hemosiderin was found in thalassemia major, with the youngest one of 5.3 years old. Early dysfunction of pancreatic exocrine and endocrine glands was found in thalassemia major, with the youngest one of 5.5 years old. Therefore, we suggest age of 5 to 6 years old as the optimal initial age for pancreatic T2* scanning.
Purpose
To determine the optimal timing of pancreatic iron screening in thalassemia major(TM) with magnetic resonance imaging (MRI) T2*.Materials and Methods
The pancreatic T2* assessments of 109 TM patients age from 5.3 to 35.0 years were retrospectively analyzed. Meanwhile, clinical data and laboratory tests results, including serum ferritin,fasting glucose, insulin, lipase and amylase performed within one week before or after MRI examination, were also collected. Correlations between pancreatic T2* and clinical findings were assessed using either Pearson or Spearman correlation tests.Weighted receiver operator characteristic (ROC) analysis was performed between pancreatic R2*, liver R2*,serum ferritin and cardiac R2* to determine how well pancreatic or hepatic iron served as a surrogate for cardiac iron deposition.Results
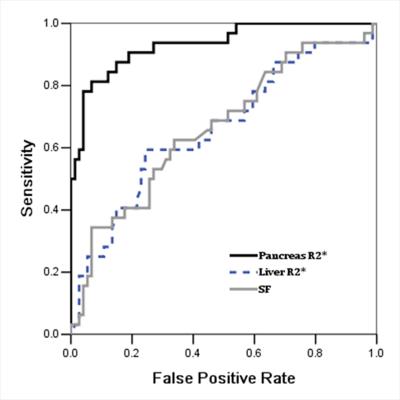
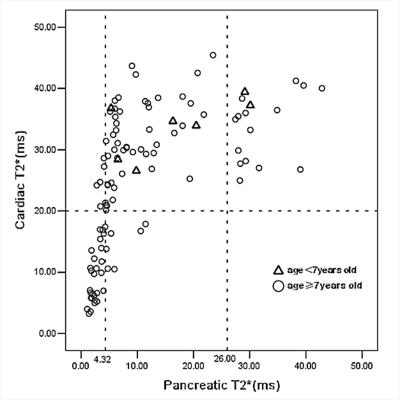
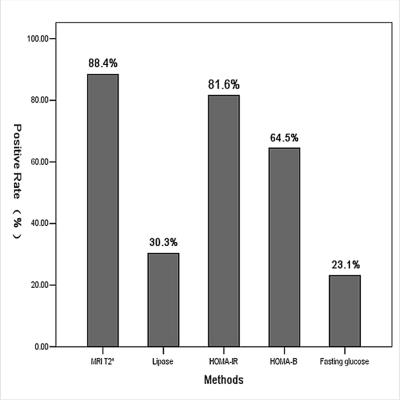
The characteristics data of all the patients are summarized in Table 1. The pancreatic T2* was positively correlated with cardiac T2* (r=0.782, p<0.001). Area under ROC curve (AUC) of pancreatic R2* for diagnosing cardiac iron overload(R2*>100Hz,T2*<20ms) was 0.933, p<0.001(Figure 1). A pancreatic R2*cut-off of 231.81Hz (pancreatic T2* of 4.32ms) led to the highest Youden’s index of 0.74, with sensitivity of 81% and specificity of 93%. With T2* cut-off of 26ms from 120 normal controls reported by Restaino G[1], 92 cases (88.4%) had detectable pancreatic iron overload, with the youngest one of 5.3 years old. Among the 92 patients, 34 cases (37%) had cardiac iron overload. Pancreatic T2* values in 5 of 7 (71.4%) patients under 7 years old were <26ms (5.3 to 6.0 years) but none was <4.32ms, while T2* values in 87 of 102 (85.3%) patients above 7 years of age were <26ms (7.0 to 35.0 years) and 32 of them(36.8%)were <4.32ms (Figure 2). As compared, the sensitivity of MRI T2* diagnosing pancreatic hemosiderin was higher than biochemical tests for pancreatic exocrine and endocrine functions (Figure 3). Among the 92 patients with pancreatic hemosiderin, 66 cases have been tested for lipase and 15 of them(22.7%) had decreased lipase level, with the youngest one of 5.5 years old (lipase =13U/L). HOMA index was more sensitive than the other biochemical tests, but still less sensitive than MRI T2*. In the follow-up study, 7 patients showed higher pancreatic T2* values over time(Figure 4), which indicated less iron overload in pancreas. T2* in one patient remained identical, T2* of the rest two patients decreased slightly.Disccusion
Iron overload is a critical systemic complication in patients with thalassemia major. The specific organ involved in the iron overload presents numerous disorders. These patients are subjected to a chelation therapy and MRI is being a noninvasive method for tailoring the chelation therapy [2-4]. Until now, studies about cardiac iron screening with MRI T2* technique suggested a range of 5 to 10 years old as the initial checking time [5-7]. Our study showed that pancreatic hemosiderin correlated with cardiac hemosiderin, and could be a predictor of the later, which was in consistent with previous studies[2, 8].Thus, it is necessary to do early screening of pancreatic hemosiderin and intensify chelation therapy accordingly may achieve double. Previous study reported that biochemical tests for pancreatic exocrine and endocrine function could be predictor of pancreatic and even cardiac iron deposition[9]. However, biochemical tests may lag behind MRI findings. Our study showed that the sensitivity of MRI T2*diagnosing pancreatic hemosiderin was higher than biochemical tests for pancreatic exocrine and endocrine functions. Intensive chelation therapy in the early stage may avoid diabetes. Platis [10] and Farmaki K[11] achieved reversal glucose metabolism disturbances by intensive combination therapy, particularly in early stages of glucose intolerance. Similarly, a majority of patients in the follow-up study had showed improved pancreatic T2* value overtime after adjustment of chelation therapy. Hence, measurement to prevent diabetes should take in early stage. A vast majority of patients in current study had pancreatic hemosiderin, with the youngest one of 5.3 years old. Nearly one third cases above 7 years old had severe pancreatic hemosiderin, while no patients under 7years old had. Therefore, an age range from 5 to 6 years old may be suitable for the first pancreatic MR T2* examination. Patients of current study could finish multi-organ MRI T2* examinations without sedation at a time, although many of them were under 9 years old with the youngest one of 5.3 years old.Conclusion
Pancreatic hemosiderin happens early in TM patients, even as young as 5.3 years old. Assessment of pancreatic hemosiderin might need to start as early as 5 to 6 years old if poor compliance is suspected or if chelation has been delayed.Acknowledgements
This work was supported by the Sun Yat-Sen University Clinical Research 5010 Program (2013004). The authors declare no competing financial interests.References
[1] Restaino G, Meloni A, Positano V, et al. Regional and global pancreatic T*2 MRI for iron overload assessment in a large cohort of healthy subjects: Normal values and correlation with age and gender[J]. Magnetic Resonance in Medicine,2011,65(3):764-769.
[2] Noetzli L J, Papudesi J, Coates T D, et al. Pancreatic iron loading predicts cardiac iron loading in thalassemia major[J]. Blood,2009,114(19):4021-4026.
[3] Noetzli L J, Panigrahy A, Mittelman S D, et al. Pituitary iron and volume predict hypogonadism in transfusional iron overload[J]. American Journal of Hematology,2012,87(2):167-171.
[4] Lam W W M, Au W Y, Chu W C W, et al. One-stop measurement of iron deposition in the anterior pituitary, liver, and heart in thalassemia patients[J]. Journal of Magnetic Resonance Imaging,2008,28(1):29-33.
[5] Borgna-Pignatti C, Meloni A, Guerrini G, et al. Myocardial iron overload in thalassaemia major. How early to check?[J]. British Journal of Haematology,2014,164(4):579-585.
[6] Chen X, Zhang Z, Zhong J, et al. MRI assessment of excess cardiac iron in thalassemia major: When to initiate?[J]. Journal of Magnetic Resonance Imaging,2015,42(3):737-745.
[7] Wood J C, Origa R, Agus A, et al. Onset of cardiac iron loading in pediatric patients with thalassemia major[J]. Haematologica,2008,93(6):917-920.
[8] Reeder S B, Pineda A R, Wen Z, et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging[J]. Magn Reson Med,2005,54(3):636-644.
[9] Yamamura J, Grosse R, Jarisch A, et al. Pancreatic exocrine function and cardiac iron in patients with iron overload and with thalassemia[J]. Pediatric Blood & Cancer,2011,57(4):674-676.
[10] Platis O, Anagnostopoulos G, Farmaki K, et al. Glucose metabolism disorders improvement in patients with thalassaemia major after 24-36 months of intensive chelation therapy[J]. Pediatr Endocrinol Rev,2004,2 Suppl 2:279-281.
[11] Farmaki K, Angelopoulos N, Anagnostopoulos G, et al. Effect of enhanced iron chelation therapy on glucose metabolism in patients with β-thalassaemia major[J]. British Journal of Haematology,2006,134(4):438-444.
Figures
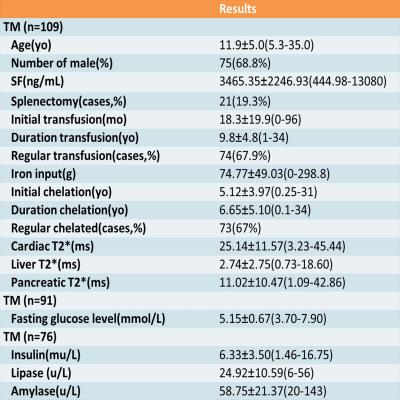
Table 1.Characteristics of 109 TM patientsa
aAll values are presented as mean±SD(range), except for the gender, splenectomy, regular transfusion and regular chelation.
TM, thalassemia major; SF, serum ferritin.