0319
Prognostic value of hepatobiliary phase MRI in patients with primary sclerosing cholangitis – Assessment of clinical outcome and evaluation of surrogate parameters1Department of Diagnostic and Interventional Radiology, Hannover Medical School, Hannover, Germany, 2Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, Hannover, Germany
Synopsis
In
this prospective study we assessed the prognostic value of hepatobiliary phase
(HBP) MRI in patients with primary sclerosing cholangitis (PSC). Relative
enhancement (RE) in the HBP after gadoxetate disodium injection correlated
significantly with clinical scores (MELD, Mayo Risk) established to estimate
survival in patients with chronic liver disease and PSC. More importantly, a
significant correlation with previously suggested surrogate parameters for
clinical outcome as well as with solid clinical endpoints (development of
tumor, liver transplantation, death) at follow-up could be observed. These
promising results attest HBP MRI in patients with PSC a potential prognostic
role and warrant further long-term evaluation.
Purpose
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease of unknown etiology, characterized by diffuse fibrosing inflammation and obliteration of the intra- and extrahepatic bile ducts. Gadoxetate disodium is a liver specific contrast agent, which shows an uptake by hepatocytes and subsequent biliary excretion of approximately 50% in patients with normal liver and renal function1. Hepatic uptake and biliary contrast excretion is known to be delayed, respectively reduced in patients with PSC and impaired liver function2. The purpose of the present study was twofold: First, to assess the value of gadoxetate disodium-enhanced MRI for evaluation of hepatic function and disease severity in patients with PSC, and secondly to correlate hepatobiliary phase (HBP) findings with clinical outcome, as determined by means of solid endpoints as well as previously suggested surrogate parameters of outcome3,4.Methods
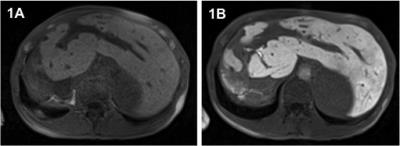
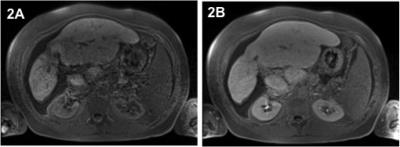
111 patients (83m/28f; mean age 45 years) with confirmed diagnosis of PSC referred for liver MRI were included in this prospective IRB-approved study. All patients underwent a clinical routine liver protocol, including T1-weighted sequences before contrast administration (pre) as well as sequences in the HBP acquired approximately 20 minutes (HBP1) and 120 minutes (HBP2) after i.v. injection of gadoxetate disodium (0.1ml/kg). Signal intensity (SI) measurements were performed in consensus by two radiologists by drawing regions of interest in each liver segment on identical positions of the corresponding datasets before and after contrast injection, and compared (Wilcoxon signed rank Test). The relative enhancement (RE) was calculated between mean pre contrast SI and HBP SI by means of the formula ((SIHBP)-SI(pre)) /(SIpre). RE at HBP1 and at HBP2 was correlated with liver function tests (LFTs) obtained within 24 hours of the MRI, as well as with the MELD, AMSTERDAM and MAYO Risk Score, respectively (Spearman). Further, RE at HBP1 and HBP2 was correlated with validated solid clinical endpoints (tumor development, liver transplantation, death) using logistic regression. In addition, further MR measurements including mean SI and interquartile range (IQR) of segmental SI pre contrast and at HBP1 and HBP2, as well as the mean SI differences between pre and HBP1 and HBP were calculated and correlated. For all measurements a p-value <0.05 was deemed significant.Results
Significant changes of hepatic SI between non-enhanced and post Gd imaging were observed in all liver segments (p<0.0001). Mean hepatic SI before gadoxetate disodium injection was 173, 306 at HBP1 and 288 at HBP2 imaging, corresponding with a mean increase of 80% (HBP1) and 72% (HBP2), respectively. A significant correlation especially of RE at HBP1 with several LFTs (e.g. with CHE p<0.0001; r=0.600) was observed, notably with the previously suggested surrogate parameters for clinical outcome alkaline phosphatase (p<0.0001; r=-0.636, serum bilirubin (p<0.0001; r=-0.646), albumin (p<0.0001; r=0.538) and INR (p<0.0001; r=-0.456). Further, RE at HBP1 correlated significantly with the MELD score (p<0.0001; r=-0.587) as well as with the MAYO Risk Score (p<0.0001; r=-0.535). Similar (but not as strong) correlations with the above-mentioned parameters were observed with RE at HBP2, mean SI at HBP1 as well as with the SI difference between pre-contrast and HBP1. Solid clinical endpoints were reached by 21 patients (tumor n=6; liver transplantation n=8; death n=7) within a mean follow-up of 603 days after MRI. In this context, a significant correlation of RE at HBP1 was observed with all endpoints: tumor development (p=0.032), liver transplantation (p=0.02) and death (p=0.027), whereas RE at HBP2 significantly correlated only with the endpoint liver transplantation (p=0.003).Discussion
PSC is a progressive cholestatic illness eventually leading to biliary cirrhosis and end-stage liver disease. MRI, especially by means of MRCP for bile duct assessment, has become a cornerstone in diagnosis and follow-up of patients with PSC. Contrast-enhanced imaging has been demonstrated to be a promising tool in this scenario especially with regards to detection of complications as well as assessment of liver function. However, the prognostic role of contrast-enhanced imaging in patients with PSC has yet to be determined. In the present study we were able to demonstrate that RE of the liver parenchyma in the HBP after gadoxetate disodium correlated not only with LFTs known to fluctuate during the course of the disease but also with clinical scores established to estimate survival in patients with chronic liver disease (MELD Score) and PSC (Mayo Risk Score). From a clinical perspective even more important, RE correlated with previously suggested surrogate parameters for clinical outcome and solid endpoints at follow-up, namely the development of tumor, event of liver transplantation and death.Conclusion
Contrast-enhanced MRI in terms of hepatobiliary phase imaging using hepatocyte-specific contrast agents has a prognostic value in patients with PSC and the promising results of our study warrant further long-term evaluation.Acknowledgements
noneReferences
1. Van Montfoort JE, Stieger B, Meijer DK, et al. Hepatic uptake of the magnetic resonance imaging contrast agent gadoxetate by the organic anion transporting polypeptide OATP1. J Pharmacol Exp Ther. 1999;290(1):153-157.
2. Hinrichs H, Hinrichs JB, Gutberlet M, et al. Functional gadoxetate disodium-enhanced MRI in patients with primary sclerosing cholangitis (PSC). Eur Radiol 2016;26(4):116-1124.
3. Ponsioen CY, Chapman RW, Chazouillères O, et al. Surrogate Endpoints for clinical trials in primary sclerosing cholangitis: Review and results from an International PSC Study Group consensus process. Hepatol 2016; 63(4):1357-1367.
4. Watanabe T, Hirano K, Tada M, et al. Short-term prognostic factors für primary sclerosing cholangitis. J Hepatobilary Pancreat Sci 2015;22:486-490.
Figures