0317
Physiologically-constrained Multiagent DCE-MRI for Pancreatic Cancer ImagingMatthias C Schabel1,2, Erin Gilbert3, Alexander Guimaraes4, and Cory Wyatt1
1Advanced Imaging Research Center, Oregon Health & Science University, Portland, OR, United States, 2Utah Center for Advanced Imaging Research, University of Utah, Salt Lake City, UT, United States, 3Surgery, Oregon Health & Science University, Portland, OR, United States, 4Radiology, Oregon Health & Science University, Portland, OR, United States
Synopsis
Physiologically-constrained multiagent pharmacokinetic modeling in pancreas using sequential injections of gadoteridol and ferumoxytol reveals differences between healthy pancreas in high-risk patients and both IPMN and pancreatic ductal adenocarcinoma.
Introduction
Pancreatic cancer remains a disease with exceptionally poor prognosis and high mortality except in rare cases when it is diagnosed early enough for complete surgical resection. While DCE-MRI has found application in imaging characterization of a wide range of malignancies, it has seen limited use in pancreatic cancer. In an attempt to increase the scope and accuracy of physiological parameters extracted from DCE-MRI data in the pancreas, we performed multiagent DCE-MRI using both a low-molecular-weight Gd-based contrast agent (gadoteridol) and an iron-based nanoparticle blood pool agent (ferumoxytol) in three cohorts of human volunteers. Resulting data were fit with a constrained multiagent model to extract physiological parameters.Methods
Multiagent DCE-MRI studies were performed in three separate cohorts of volunteers, including 10 patients with high risk for pancreatic cancer, 4 patients with IPMN lesions, and 5 patients with pancreatic ductal adenocarcinoma (PDAC). After initial pre-contrast imaging, including non-contrast MRCP, patients were scanned with a fast 3D SPGR (TR<3 ms, TE<1 ms, flip angle = 17 degrees, 3 mm isotropic spatial resolution, tacq<4 sec/frame) acquisition in the coronal plane, including both the entirety of the pancreas and the descending aorta during sequential gadoteridol and ferumoxytol injections. Arterial input functions were determined by fitting measured data from hand-drawn ROIs of the aorta between the level of the renal arteries and the iliac bifurcation. Regions of interest containing “normal” pancreas and “abnormal” pancreas were identified by a radiologist from post-contrast VIBE images in each data set. Curves of relative signal enhancement were extracted and each bolus in the resulting contrast-uptake data was simultaneously fit with a six-parameter Gamma Capillary Transit Time (GCTT) model [1]. This model is a generalization of other models such as TK, ETK, ATH, and 2CX that incorporates finite vascular transit time and heterogeneity in the distribution of vascular transit times, with free parameters for interstitial fraction (fe=ve/(1-vb)), blood volume (vb), first pass extraction fraction (E), transit time (tc), vascular heterogeneity index (1/alpha), and delay time (td). By imposing physiological constraints across the two boluses, twelve free parameters across two agents are reduced to a total of seven free parameters [2]. The decrease in model complexity achieved through application of these physiological constraints results in self-consistent parameter estimates with much improved modeling consistency.Results
Figure 1 shows measurements and constrained model fits to representative multiagent data averaged over the whole pancreas in a healthy high-risk subject (panel A), averaged over the “normal” pancreas (blue) and lesion (red) in an IPMN patient (panel B), and averaged over the “normal” pancreas (blue) and tumor (red) in a PDAC patient (panel C). Figure 2 shows box plots of parameter values for different patient groups, with parameters with statistically-significant deviations from the values in the healthy HR patient group indicated by * (p<0.05) or ** (p<0.01).Discussion
Statistically-significant differences were observed between our high-risk patient cohort and both IPMN and PDAC cohorts. In particular the strongest statistical findings were: fe higher (p<0.01) in both radiologically-normal appearing pancreas and tumor in PDAC patients, and kep lower (p<0.01) in IPMN lesion) and PDAC tumor (p<0.01). Other differences appeared at the weaker p<0.05 level. Extraction fraction for ferumoxytol was generally an order of magnitude or more smaller than that for gadoteridol, demonstrating that the constrained-modeling approach produces physically-reasonable results.Conclusion
We have demonstrated for the first time that it is feasible to perform multiagent DCE-MRI with physiologically-constrained pharmacokinetic modeling in human studies. The resulting parameters show significant differences between high-risk patients and those with IPMN or PDAC, suggesting that this approach may be of utility in identifying pancreatic malignancies via in vivo imaging.Acknowledgements
This work was funded by the Brenden-Colson Center for Pancreatic Care.References
1. Schabel MC, MRM (2012) 68(5):1632-1646. A unified impulse response model for DCE-MRI.
2. Jacobs I, et al., MRM (2016) 75(3):1142-1153. A novel approach to tracer-kinetic modeling for (macromolecular) dynamic contrast-enhanced MRI.
Figures
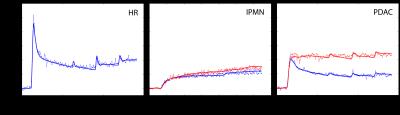
Figure 1: Multiagent DCE-MRI data and constrained
model fits in pancreas. Relative enhancement is
plotted against measurement time for a high-risk patient (panel A), a patient
with IPMN (panel B, “normal” pancreas in blue, lesion in red), and a patient
with PDAC (panel C, “normal” pancreas in blue, tumor in red).
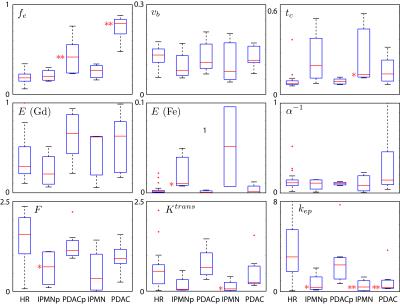
Figure 2: Box plots of constrained model parameters. Model parameters for the entire
pancreas from high-risk patients (HR) are compared with
values from IPMN “normal” pancreas (IPMNp), PDAC “normal” pancreas (PDACp),
IPMN lesion (IPMN), and PDAC tumor (PDAC) ROIs. Statistically significant
differences between HR and other distributions are indicated by * (p<0.05)
or ** (p<0.01).