0315
Magnetisation Transfer MRI Facilitates Non-Invasive Identification of Fibrosis in Chemically-Induced Rat Mammary Carcinomas Imaged on a 1.5T Clinical Platform1CRUK Cancer Imaging Centre, Division of Radiotherapy and Imaging, The Institute of Cancer Research, London, United Kingdom, 2Department of Radiology, Royal Marsden NHS Foundation Trust, London, United Kingdom
Synopsis
Intratumoural fibrosis is associated with poor prognosis in breast cancer patients. Non-invasive detection of such fibrosis may contribute to the provision of personalised treatment regimens. Multi-parametric MRI, using a clinical MRI scanner and incorporating endogenous contrast mechanisms, was performed on MNU-induced rat mammary carcinomas to identify parameters sensitive to the detection and quantification of fibrosis. Magnetisation transfer MRI derived parameters correlated with percentage picrosirius red staining, which detects collagen I/III, major components of fibrosis, in this heterogeneous tumour cohort. These results strongly support the inclusion of magnetisation transfer in clinical MR breast imaging protocols.
Introduction
Pathologically-confirmed fibrosis is associated with poor prognosis in breast cancer;1 invasive ductal carcinomas with a central scar-like fibrotic focus created by exaggerated reactive tumour stroma have been shown to be more aggressive than those without2 and presence of a fibrotic focus is a predictor of early distant breast cancer relapse.3 The ability to non-invasively detect breast tumour fibrosis would contribute to the provision of personalised treatment regimens.
Multi-parametric MRI incorporating several endogenous contrast mechanisms was assessed for its ability to detect and quantify fibrosis in a chemically-induced rat model of mammary carcinoma.
Methods
Female Sprague Dawley rats (n=18) were injected with 37.5 mgkg−1 of refrigerated N-methyl-N-nitrosourea (MNU) intraperitoneally, resulting in tumours that developed at various sites associated with the mammary pad.4 When tumours reached ~3 cm3, rats were imaged on a clinical 1.5T scanner (Siemens Healthcare) using a head/neck array coil in parallel with a small-loop temporomandibular joint coil. Following the acquisition of T2-weighted coronal images for tumour localisation, prototype diffusion-weighted MRI (DWI), prototype ultrashort echo time (UTE), and magnetisation transfer (MT) MRI data were acquired over the entire tumour using the sequence parameters in Table 1.
Following MRI, tumours were excised, formalin-fixed, and paraffin-embedded with orientation matching the imaging plane. 5 µm sections through the tumour centre were cut and stained: H&E staining was assessed by an expert pathologist to determine tumour grade. Picrosirius red (PR) staining for collagen I/III, major components of fibrosis, was estimated as a percentage of each slice using semi-automatic L*a*b space colour separation (MATLAB).
The MRI slice macroscopically matched to the histology was assessed, and tumour ROIs analysed on a voxel-by-voxel basis. DWI data were analysed as described previously5 to provide ADC and IVIM parameters f, D, D* and fD*. UTE data were analysed for ultrashort TE echoes (T2*short, TE<1 ms), and for conventional gradient-echo echoes (T2*long, TE>1 ms) separately using single exponential models. MT data was analysed using the methods of Helms and Scholz6,7 to derive the magnetisation transfer ratio (MTR), MT parameters ka, the apparent rate of magnetisation transfer, and δ, the propensity for water signal destruction by the MT pulse, and variable-flip-angle T1 estimates for the voxel with and without the saturation pulse (T1 and T1s, respectively), and of the unbound component of the water signal (T1free). Pearson correlation of MR parameters with PR staining were calculated, with p<0.0125 considered significant to correct for multiple comparisons (Bonferroni correction) not described herein.
Results
Sixteen matched MRI and histological datasets from thirteen tumours in twelve rats (one animal with two tumours, and two distinct regions each from three tumours) were analysed; MR images and histological assessment demonstrated substantial inter-tumoural heterogeneity (Figure 1). Time from MNU injection to imaging (days), tumour volume, grade, and percentage PR staining are shown in Table 2. MRI parameters were plotted against percentage PR staining for each section: from DWI and UTE acquisitions, correlations with PR staining were statistically significant for the pseudodiffusion parameter fD* (range 7.5 to 48.7x10-4 mm-2s, r=0.806) and T2*long (range 16.5 to 58.3 ms, r=-0.668). Significant correlations with PR staining were found for MT parameters T1 (range 0.80 to 1.59 s), T1s (range 0.3 to 0.66 s), T1free (range 0.7 to 1.2 s), ka (range 0.7 to 1.8 s-1) and δ (range 1.0 to 4.2 %) (Figure 2). The correlation with MTR did not reach statistical significance (range 44.1 to 63.8 %, p=0.019).Discussion
Chemically-induced mammary carcinomas arising in rats injected with MNU were highly heterogeneous and presented with a range of fibrosis levels. MRI parameters derived from magnetisation transfer measurements correlated with the level of collagen I/III deposition (fibrosis) histologically observed in these tumours. A reduction in all T1 parameters with increasing amounts of fibrous collagen was observed in these tumours. Further, ka and δ were positively correlated with collagen and demonstrate the increased MT effect (exchange of the water proton magnetisation) with increased presence of collagen, which is mirrored in the (non-significant) correlation observed for the simple ratio MTR. Data obtained from UTE and DWI data were less informative, although showed a possible sensitivity for the parameters T2*long, obtained from multiple gradient echo acquisitions, and the pseudo-diffusion parameter fD*.Conclusion
These results from a preclinical model of mammary carcinoma, performed using clinical hardware and imaging sequences, clearly demonstrate the ability of magnetisation transfer imaging contrast to assess the presence of intratumoural collagen, and strongly support the inclusion of MT for clinical MR breast imaging. MT-MRI may also prove useful for the assessment of radiation-induced fibrosis in the breast, a delayed side effect of radiotherapy.Acknowledgements
CRUK and EPSRC support to the Cancer Imaging Centre at The Institute of Cancer Research and The Royal Marsden Hospital in association with the MRC and Department of Health (England) (C1060/A10334, C1060/A16464) and NHS funding to the NIHR Biomedical Research Centre and the Clinical Research Facility in Imaging at The Royal Marsden and the ICR. We thank Dr. Berthold Kiefer of Siemens Healthcare, Erlangen, Germany, for provision of the prototype MR sequences used in this work.
References
1. Van den Eynden GG, Colpaert CG, Couvelard A, et al. A fibrotic focus is a prognostic factor and a surrogate marker for hypoxia and (lymph) angiogenesis in breast cancer: review of the literature and proposal on the criteria of evaluation. Histopathology. 2007;51(4):440–451.
2. Hasebe T, Tsuda H, Hirohashi S, et al. Fibrotic focus in invasive ductal carcinoma: an indicator of high tumor aggressiveness. Jpn J Cancer Res 1996;87:385–94.
3. Colpaert C, Vermeulen P, Jeuris W, Early distant relapse in ‘node-negative’ breast cancer patients is not predicted by occult axillary lymph node metastases, but by the features of the primary tumour. J Pathol. 2001;193(4):442-9.
4. McPhail LD, Robinson SP. Intrinsic susceptibility MR imaging of chemically induced rat mammary tumors: relationship to histologic assessment of hypoxia and fibrosis. Radiology. 2010;254(1):110-118.
5. Jerome NP, Boult JK, Orton MR, et al. Modulation of renal oxygenation and perfusion in rat kidney monitored by quantitative diffusion and blood oxygen level dependent magnetic resonance imaging on a clinical 1.5T platform. BMC Nephrol. 2016;17(1):142.
6. Helms G, Dathe H, Kallenberg K, Dechent P. High-resolution maps of magnetization transfer with inherent correction for RF inhomogeneity and T1 relaxation obtained from 3D FLASH MRI. Magn Reson Med. 2008;60(6):1396-1407.
7. Scholz TD, Ceckler TL, Balaban RS. Magnetization transfer characterization of hyperintensive cardiomyopathy: significance of tissue water content. Magn Reson Med. 1993;29(3):352-7.
Figures
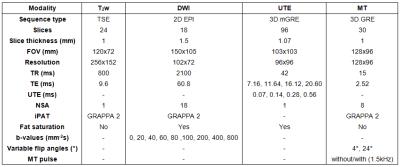
Table 1: MRI sequence parameters.
MRI performed using a clinical 1.5T magnet, using a parallel array of clinical coils (head/neck array coil with a small-loop temporomandibular joint coil). All sequences were adapted from clinical product sequences.
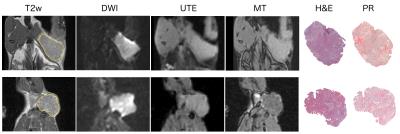
Figure 1.
Example MR and histological images acquired from two MNU-induced rat mammary carcinomas. Tumour regions of interest are shown on T2-weighted (T2w) images. MR images shown: Diffusion-weighted imaging (DWI) b=0 mm-2s, ultrashort echo time (UTE) TE=7.16 ms, magnetisation transfer (4° with MT pulse). Whole section images of H&E and picrosirius red (PR) stained FFPE tumour sections show tissue architecture and collagen I/III deposition, respectively.
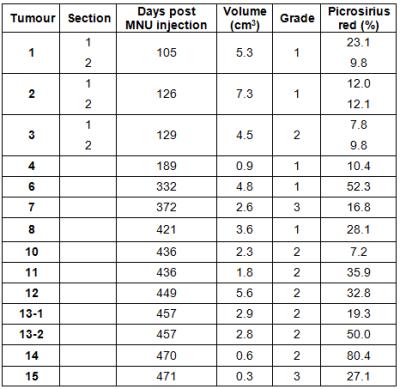
Table 2: Cohort characteristics for MNU-induced mammary carcinomas propagated in rats.
Two distinct regions of three tumours were assessed (1-3) and two separate tumours were assessed from rat 13 (13-1 and 13-2). Tumour volume was calculated using segmentation on T2-weighted MRI images.
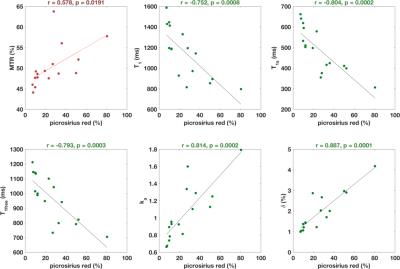
Figure 2.
Correlations between magnetisation transfer imaging parameters and percentage picrosirius red staining on a tumour-by-tumour basis. Pearson correlation coefficient (r) was calculated and p<0.0125 considered significant. MTR = magnetisation transfer ratio.