0309
Multiparametric MR for assessment of tissue characteristics of small intestine neuroendocrine tumour evaluated by histological correlations1Department of Radiation Physics, University of Gothenburg, Gothenburg, Sweden, 2Department of Pathology, University of Gothenburg, Gothenburg, Sweden
Synopsis
This study investigates the relations between MR derived, quantitative parameters reflecting perfusion, diffusion and relaxation, and histological indices reflecting apoptosis, proliferation, vascularity and fibrosis. We show that important biological characteristics of tumour tissue can be probed by multiparametric MRI.
Introduction and Purpose
Modern cancer therapies are designed to target e.g. tumour angiogenesis, proliferation and evasion of apoptosis1. To facilitate early response assessment, the methods used should be sensitive to these biological processes and preferably be non-invasive. Multiparametric MRI, i.e. several MR-methods in one examination, fulfils these criteria and gives information about the entire tumour2. However, before multiparametric MRI can be used for comprehensive characterisation of tumour tissue, the understanding of the associations between histological features and MR-parameters must be improved.
The aim of this study was to investigate the relation between MRI-parameters and histological indices in neuroendocrine tumours after 177Lu-octreotate therapy, and thereby identify potential MRI-based biomarkers for early tumour therapy response assessment.
Material and Methods
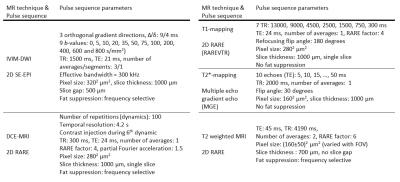
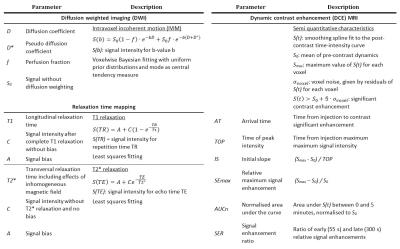
MRI of five mice with subcutaneous human small intestine neuroendocrine tumour, GOT1, was performed using a 7T Bruker MR system. The examinations included intravoxel incoherent motion diffusion weighed imaging (IVIM-DWI), T1- and T2*-mapping, dynamic contrast enhancement (DCE) MRI and T2-weighted MRI (cf. fig. 1). Model based and semi-quantitative parameters were calculated (cf. fig. 2).
After 177Lu-octreotate administration on day 0, MR examinations were performed on day 1 (all techniques in fig. 1, animal 1) or day 8 (DCE-MRI, animals 2-5) and 13 (all techniques except DCE-MRI, animals 2-5). Different days were used to increase the variability in tissue response characteristics.
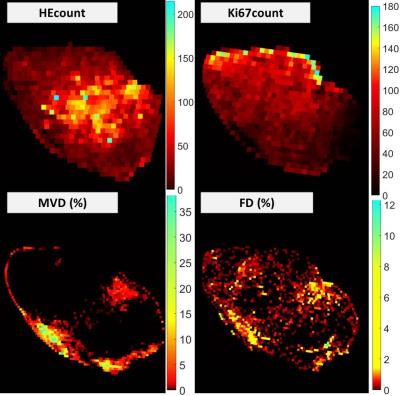
Animals were killed directly after the MR examination and tumour tissue was harvested for histological analyses. Four histological slices from the most centrally imaged tumour section were stained using haematoxylin (HE), Ki67, CD31 and Masson Trichrome (MT), and digitalized using a Leica Slide Scanner (0.25x0.25µm2 resolution). Histological indices were calculated from 250x250μm2 regions in images. Indices reflecting apoptosis (HEcount) and proliferation (Ki67count) were based on automatic cell counting, whereas those reflecting micro-vascular density (MVD) and fibrotic density (FD) were based on fractional stained areas after colour segmentation on CD31 and MT stained sections, respectively, and results were validated by a pathologist.
Maps of histological indices and MR-parameter were fused by landmark-based local registration. Only regions with distinct landmarks in both MR and histological images yielding adequate registrations quality were further analysed. Associations between histological indices and MR-parameters were evaluated pairwise using linear mixed effects regression, which accounts for possible variations in e.g. staining between tumours.
Results and Discussion
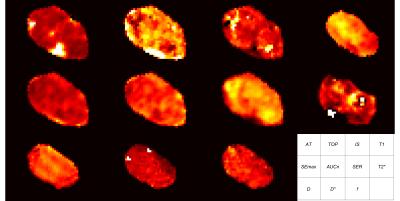
In total, data could be extracted from 1821 regions in the five examined tumours. The calculated maps showed heterogeneous patterns, differing between the various MR-parameters (fig. 3) and histological indices (fig. 4).
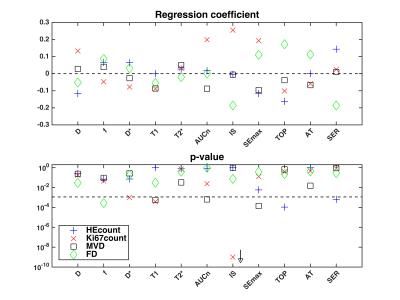
The linear mixed effects regression showed that all histological indices were linearly related to at least one MR-parameter on a statistically significant level (fig. 5).
The negative/positive correlation between HEcount and exchange rate parameter TOP/SER may imply increased apoptosis in regions of adequate vascular function and perfusion, allowing successful delivery of the 177Lu-octreotate. This may also explain the positive correlation between f and FD, since fibrosis has been observed after successful treatment in this tumour model.
Highest statistical significance was found in a strong, positive correlation between IS and Ki67count. Assuming IS describes perfusion3,4, it is reasonable that regions with high IS show higher cell proliferation, due to adequate supply of nutrients and oxygen. Estimates of D* were hampered by poor quality and the correlation with Ki67count should not be interpreted. The negative correlation between T1 and Ki67count may be explained by increased interaction between water hydrogen and cell membranes when cell numbers increase, which in turn shortens T15.
T1 was also negatively correlated with MVD. This is in agreement with the increased T1 observed after therapies inhibiting angiogenesis6-8. Angiogenesis is induced by increased levels of vascular endothelial growth factor (VEGF), which increases MVD, but also micro-vessel permeability8. Plasma proteins may thus leak to the interstitial space and accumulate due to poor lymphatic drainage in tumours. MVD was also negatively correlated with the contrast amount-related parameters AUCn and SEmax, possibly due to increased permeability, and thereby increased interstitial fluid pressure (IFP). When IFP reach micro-vascular pressure levels effectively hinder perfusion and extravasation of e.g. (radio-)pharmaceuticals to the interstitial space, and thereby to the tumour cells8.
Neither T2* nor D showed associations with histology in the present study, perhaps due to small variations in tissue characteristics in the current data set.
Conclusion
This work emphasizes the importance of including multiple MR techniques for monitoring of tumour tissue characteristics. Eight MR-parameters were significantly correlated with histological indices reflecting apoptosis, proliferation, micro-vascular density and fibrosis in the studied tumour type, and should be further evaluated as potential imaging biomarkers for therapy response assessment.Acknowledgements
This study was supported by grants from the Swedish Research Council, the Swedish Cancer Society, BioCARE – a National Strategic Research Program at the University of Gothenburg, the King Gustav V Jubilee Clinic Cancer Research Foundation, the Sahlgrenska University Hospital Research Funds, the Assar Gabrielsson Cancer Research Foundation, the Adlerbertska Research Fund, the Wilhelm and Martina Lundgren science trust fund and the Royal Society of Arts and Sciences in Gothenburg (KVVS).References
1. Figueiras RG, Padhani AR, Goh VJ, et al. Novel oncologic drugs: what they do and how they affect images. Radiographics : a review publication of the Radiological Society of North America, Inc. 2011;31:2059-2091.
2. Dominietto M, Rudin M. Could magnetic resonance provide in vivo histology? Frontiers in genetics. 2014;4:298.
3. Li LZ, Zhou R, Xu HN, et al. Quantitative magnetic resonance and optical imaging biomarkers of melanoma metastatic potential. Proceedings of the National Academy of Sciences. 2009;106:6608-6613.
4. Rudin M. Imaging readouts as biomarkers or surrogate parameters for the assessment of therapeutic interventions. European Radiology. 2007;17:2441-2457.
5. Jakobsen I, Lyng H, Kaalhus O, et al. MRI of human tumor xenografts in vivo: Proton relaxation times and extracellular tumor volume. Magnetic Resonance Imaging. 1995;13:693-700.
6. Ravoori MK, Nishimura M, Singh SP, et al. Tumor T1 Relaxation Time for Assessing Response to Bevacizumab Anti-Angiogenic Therapy in a Mouse Ovarian Cancer Model. PloS one. 2015;10:e0131095.
7. Lescher S, Jurcoane A, Veit A, et al. Quantitative T1 and T2 mapping in recurrent glioblastomas under bevacizumab: earlier detection of tumor progression compared to conventional MRI. Neuroradiology. 2015;57:11-20.
8. Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31:2205-2218.
Figures