0299
A Novel Approach to Prospective Motion Correction Using Multi-Slice-to-Volume Registration1MR Physics, Fraunhofer MEVIS, Bremen, Germany
Synopsis
This study introduces a novel 2D-EPI-navigated prospective motion correction technique to correct for in-plane and through-plane motion that establishes a flexible steady-state during the measurement. It utilizes a rigid-body multi-slice-to-volume registration using three parallel and well-separated EPI slices. The technique was evaluated using a well-defined motion protocol with translations up to 13mm and rotations up to 9°, which was executed by a volunteer. Results show a substantial reduction of motion parameters to below ±0.5mm/±0.5° and an increase in overall image quality in comparison to a no-motion scan. The navigator acquisition scheme can be adapted for use with a range of multi-shot 2D sequences to allow EPI navigators to be acquired with limited effect on the overall scan time or the contrast-to-noise ratio.
Purpose
Patient motion remains a difficult challenge in multi-shot 2D sequences. Prospective motion correction can enhance image quality by determining motion parameters during the scan and adapting the imaging system in real time. These methods typically use navigator data for motion detection, including orthogonal PROMO navigators1, cloverleaf navigators2 or volumetric EPI navigators3. However, navigator acquisitions can have significant influence on the magnetization of the sequence. This study introduces a novel 2D-EPI-navigated prospective motion correction procedure that establishes a flexible steady-state during the measurement. It corrects for in-plane and through-plane motion and offers high temporal resolution by utilizing a rigid-body multi-slice-to-volume registration using three parallel EPI slices.Methods
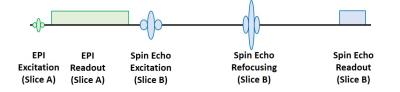
A single 2D-EPI slice acquisition is applied prior to each spin excitation of a T2-weighted spin-echo sequence to serve as navigator for rigid-body motion correction (fig. 1). To avoid direct saturation of the spin-echo signal, the slice iteration schemes for these two constituents of the sequence are decoupled.
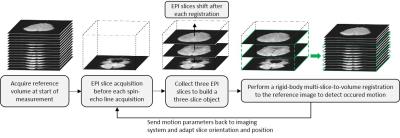
At the start of the scan, the first EPI acquisitions from each slice position are collected as reference volume (fig. 2). The subsequent slices create a series of three-slice objects of parallel, well-separated EPI slices used for a multi-slice-to-volume registration to the reference volume, which is based on the Mattes’ Mutual Information metric4 (ITK, open-source). The detected motion parameters are sent back to the sequence in real time to adapt the slice position and orientation of subsequent scans.
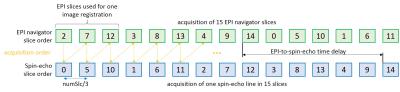
The slice acquisition order is controlled to: (1) create the required EPI three-slice objects and (2) create a steady-state which has low influence on both sequence parts. Figure 3 shows the slice iteration order for one phase-encoding line of a 15-slice spin-echo acquisition. The sequence uses an interleaved slice order with steps of one third of the slice number to create the three-slice objects. The time delay between EPI and spin-echo slice iteration can be adapted to change the steady-state and reduce saturation problems.
The method was evaluated at 3T (MAGNETOM Skyra, Siemens Healthcare GmbH) with a well-defined motion protocol, executed by a volunteer in two measurements, one with and one without prospective motion correction. An additional no-motion scan was measured for comparison. Sequence parameters were: TR=3500ms, TE=90ms, 21 slices (3mm) with 30% slice gap, 230mm FOV and 192x192 resolution. The EPI navigators had a 64x64 resolution with 680μm echo spacing. A 1-2-1 binomial pulse5 with 20° flip angle was used to suppress fat signal in the navigators. The EPI slice acquisition time was 48ms.
Results
In the 11:17 minutes measurement time, 1330 three-slice objects were acquired after the reference volume; one object every 0.5s; average time for image registration was 76.73ms.
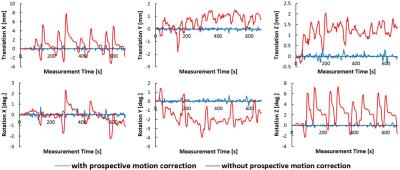
The residual motion was estimated retrospectively by registering full volumes, built from the navigator data (fig. 4). The red curves (no motion correction) show the motion parameters caused by the executed motion protocol. The volunteer moved up to 13mm in translation and 9° in rotation in a repeating fashion. The blue curves (with motion correction) show residual motion parameters well below ±0.5mm/±0.5°, despite the substantial subject motion.
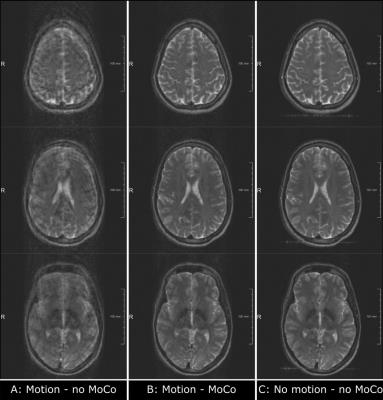
The resulting spin-echo images without applied prospective motion correction (fig. 5A) show the level of blurring, ghosting and ringing artefacts, which is typically seen when scanning uncooperative patients. With prospective motion correction (fig. 5B) artefacts are reduced to a level that would permit reliable clinical diagnosis. Compared to the no-motion scan (fig. 5C) the motion-corrected scan produced images with a slight decrease in SNR and a low-level incoherent ghost, but the overall image quality was comparable.
Discussion
The images in figure 5 show a typical problem with prospective motion correction where motion during the navigator acquisition cannot be corrected properly, resulting in low-level residual motion artefacts. Image quality can be further increased by reducing the acquisition time of the navigator, for example by using a simultaneous multi-slice acquisition to acquire all three EPI navigator slices with a single multiband excitation pulse. In combination with an accelerated registration procedure, the proposed multi-slice-to-volume technique could achieve a temporal resolution to rival that achieved with camera-based methods.Conclusion
This study has introduced a novel approach to prospective motion correction that uses three EPI slices with variable slice positions as navigators to compute a multi-slice-to-volume registration. The results show a substantial reduction for in-plane and through-plane motion parameters and demonstrate a high level of image quality in the presence of subject motion. The slice-ordering scheme can be adapted for use with a range of multi-shot 2D sequences to allow EPI navigators to be acquired with limited effect on the overall scan time or the contrast-to-noise ratio.Acknowledgements
The authors are grateful to Matthias Günther at Fraunhofer MEVIS for valuable discussions and support.
All funding for this study was provided by the internal Attract funding program of the German Fraunhofer-Gesellschaft.
References
1. White N, Roddey C, Shankaranarayanan A, et al. PROMO: Real-time prospective motion correction in MRI using image-based tracking. Magnetic Resonance in Medicine. 2010;63:91–105.
2. van der Kouwe A, Benner T, Dale A. Real-time rigid body motion correction and shimming using cloverleaf navigators. Magnetic Resonance in Medicine. 2006;56:1019–1032.
3. Bhat H, Tisdall M, Cauley S, et al. Simultaneous multi-slice (SMS) accelerated EPI navigators for prospective motion correction in the brain. Proc. Intl. Soc. Mag. Reson. Med. 2015;23:0817.
4. Mattes D, Haynor D, Vesselle H, et al. PET-CT image registration in the chest using free-form deformations. IEEE Transactions in Medical Imaging. 2003;22:120-128.
5. Hore P. Solvent suppression in fourier transform nuclear magnetic resonance. Journal of Magnetic Resonance. 1983;55:283-300.
Figures