0286
Diffusion compartment imaging reveals microstructural injuries in a mouse model of mild traumatic brain injury1Department of Radiology, Boston Children's Hospital, Harvard Medical School, Boston, MA, United States, 2Division of Emergency Medicine, Boston Children's Hospital, Harvard Medical School, Boston, MA, United States
Synopsis
Although about 30% of patients with mild traumatic brain injury (mTBI) suffer prolonged symptoms after injury1, conventional anatomic magnetic resonance imaging (MRI) has not proven useful in diagnosing or predicting outcomes after mTBI. In this work we evaluated a novel technique, diffusion compartment imaging (DCI), with a mouse model of mTBI that enables study of mTBI under controlled conditions. We compared DCI and diffusion tensor imaging (DTI) changes to histopathological observations in two injury conditions (with and without persistent functional deficits). Our results suggest that, unlike DTI, DCI detects specific evidence of traumatic axonal injury. Moreover, DCI detects changes only in mice with persistent functional deficits.
Introduction
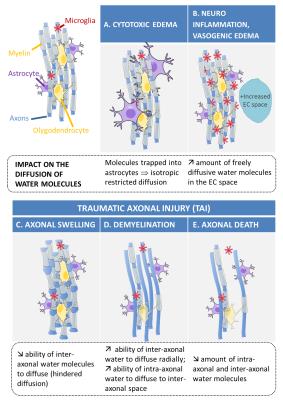
While diffusion tensor imaging (DTI)2 has proven sensitive to detecting microstructural tissue changes in mTBI in-vivo,3 it only provides a coarse summary of the diffusion in voxels and has poor specificity in describing tissue injury, as very different combinations of microstructural features may produce the same changes in DTI parameters.4 In contrast, diffusion compartment imaging (DCI) teases apart the various components of diffusion within voxels5,6 and offers the potential for sensitive and specific characterization of axonal injury, axonal death, neuroinflammation, and edema after mTBI (Fig.1). We evaluated DCI with a weight-drop mouse model of mTBI which can be graded to produce transient or prolonged functional deficits similar to clinical mTBI.7,8 We assessed changes of DCI and DTI parameters before and in the acute phase after injury in two injury conditions (mTBI with transient deficits [TD] or prolonged deficits [PD]) and compared DCI and DTI changes to histopathological evidence of injury.Methods
Mice underwent mTBI using the weight-drop model7 of mTBI and two injury conditions: 6 mice with TD mTBI and 6 mice with PD mTBI. In-vivo MRI was performed before and in the acute phase (<24h) after injury using a Bruker BioSpec 70/30 system. We acquired a T2 RARE anatomical scan (resolution= $$$0.08\times0.08\times0.5\mathrm{mm}^3$$$, TE/TR=9ms/3s, RARE factor=8, 8 averages, <10min) and a multi-shell DW-MRI scan (5 shells at $$$b=250,500,750,1000,2000\mathrm{s}/\mathrm{mm}^2$$$, multi-shot 2D EPI, 4 shots, resolution=$$$0.16\times0.16\times0.5\mathrm{mm}^3$$$, TE/TR=36ms/2500ms, 6 repetitions, <1h20). After acquisition the mice were sacrificed and the brains collected for histopathology. Immunostaining was performed for APP.
For each mice, pre- and post-injury DW scans were aligned to a common reference system defined by the pre-injury T2 scan. A template was created by manually segmenting the corpus callosum (CC) in one mouse. The CC was then automatically segmented in all scans by using non-rigid registration of the template to each mouse, and the mean of DTI and DCI parameters assessed in the corresponding region. DCI was achieved using the DIAMOND model.10 DIAMOND is a hybrid biophysical and statistical approach that characterizes each diffusive environment in each voxel using a continuous statistical distribution of diffusion tensors. It enables the assessment of compartment-specific diffusion characteristics (compartment FA, RD and MD, ie cFA,cRD,cMD) and of the intra-compartment microstructural heterogeneity (compartment heterogeneity index, cHEI). We considered a DIAMOND model with 1) one anisotropic compartment; 2) a free diffusion compartment ($$$D_\mathrm{free}=3\times10^{-3}\mathrm{mm}^2/\mathrm{s}$$$); and 3) an isotropic restricted diffusion compartment ($$$D_\mathrm{{isoR}}=1\times10^{-3}\mathrm{mm}^2/\mathrm{s}$$$). The fractions associated with the compartments were not constrained to sum to 1. Statistical significance testing was achieved using the Kruskal-Wallis test, correcting for multiple comparison to account for the number of tested parameters.
Results
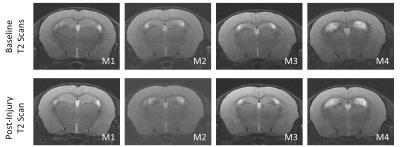
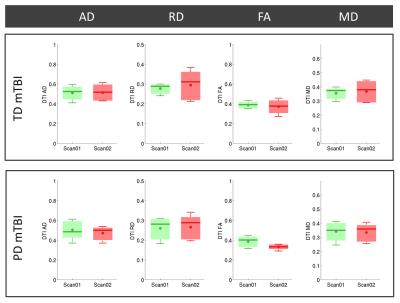
Fig.2 shows that there was no evidence of gross anatomical finding after injury. On DTI, there were no statistically significant differences before and after injury in either injury group, although a trend of decreased FA was observed after PD mTBI (Fig.3). In contrast, we found statistically significant increased isotropic restricted diffusion and increased cFA (p<0.05,Fig.4) with DCI after PD mTBI. A trend of decreased cAD, decreased cRD and increased cHEI was also observed. There was no evidence of DCI changes after TD mTBI. Fig.5 show a stronger APP expression in the CC in PD mTBI suggesting axonal injury.11Discussion
We found that DCI, compared to DTI, demonstrated increased sensitivity and specificity to microstructural injury after mTBI. DCI, but not DTI, reliably identified more severely injured mice, at risk for prolonged deficits. DCI found a decreased cFA consistent with histologically confirmed axonal injury12 (Fig.5) and evidence of increased restricted isotropic diffusion, which is consistent with cytotoxic edema that is characterized by intracellular water accumulation in astrocytes due to ionic pump failure13 (giving rise to a population of water molecules whose diffusivity is isotropic and restricted). In contrast, no such changes were evident even in more severely injured animals on DTI, which conflates the contributions from anisotropic diffusion, free diffusion and isotropic restricted diffusion in voxels. Moreover, the trend of decreased DTI FA after injury is at odd with the expectation that axonal swelling, an early stage of axonal injury, reduces the ability of water molecule to diffuse axially and radially in the extra-axonal space12 (Fig.1) and leads to increased diffusion anisotropy.Conclusion
By disentangling the various components of the diffusion within voxel, DCI improves the sensitivity of detecting injury and increases the specificity in describing microstructural changes. DCI was able to identify PD mTBI based on evidence of axonal injury in the acute phase after mTBI. DCI may provide the critically needed diagnosis tool to more accurately characterize the nature, severity and stage of injury in mTBI.Acknowledgements
This work was supported in part by grants from the NIH (U01 NS082320; R01 NS079788; R01 EB013248; R01 EB019483; and R21 EB012177) and grants from Boston Children's Hospital (Clinical and Translational Research Executive Committee (CTREC) K-to-R Merit Award; Translational Research Program (TRP) Pilot Award; and TRP Innovator Award).References
1.Bigler, E.D., Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J Int Neuropsychol Soc, 2008. 14(1): p. 1-22.
2.Basser, P.J., J. Mattiello, and D. LeBihan, MR diffusion tensor spectroscopy and imaging. Biophys J, 1994. 66(1): p. 259-67.
3.Hulkower, M.B., D.B. Poliak, S.B. Rosenbaum, et al., A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol, 2013. 34(11): p. 2064-74.
4.Jones, D.K., T.R. Knosche, and R. Turner, White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage, 2013. 73: p. 239-54.
5.Stanisz, G.J., A. Szafer, G.A. Wright, et al., An analytical model of restricted diffusion in bovine optic nerve. Magn Reson Med, 1997. 37(1): p. 103-11.
6.Latour, L.L., K. Svoboda, P.P. Mitra, et al., Time-dependent diffusion of water in a biological model system. Proc Natl Acad Sci U S A, 1994. 91(4): p. 1229-33.
7.Mannix, R., J. Berglass, J. Berkner, et al., Chronic gliosis and behavioral deficits in mice following repetitive mild traumatic brain injury. Journal of neurosurgery, 2014: p. 1-9.
8.Kondo, A., K. Shahpasand, R. Mannix, et al., Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature, 2015. 523(7561): p. 431-6.
9.Salvador, R., A. Pena, D.K. Menon, et al., Formal characterization and extension of the linearized diffusion tensor model. Hum Brain Mapp, 2005. 24(2): p. 144-55.
10.Scherrer, B., A. Schwartzman, M. Taquet, et al., Characterizing brain tissue by assessment of the distribution of anisotropic microstructural environments in diffusion-compartment imaging (DIAMOND). Magn Reson Med, 2016. 76(3): p. 963-977.
11.Johnson, V.E., W. Stewart, and D.H. Smith, Axonal pathology in traumatic brain injury. Exp Neurol, 2013. 246: p. 35-43.
12.Bazarian, J.J., T. Zhu, B. Blyth, et al., Subject-specific changes in brain white matter on diffusion tensor imaging after sports-related concussion. Magn Reson Imaging, 2012. 30(2): p. 171-80.
13.Unterberg, A.W., J. Stover, B. Kress, et al., Edema and brain trauma. Neuroscience, 2004. 129(4): p. 1021-9.
Figures