0274
High-resolution MR vessel wall imaging after intra-arterial treatment for acute ischemic stroke1Radiology, UMC Utrecht, Utrecht, Netherlands, 2Neurology and Neurosurgery, UMC Utrecht, Utrecht, Netherlands
Synopsis
Intra-arterial treatment (IAT) may damage the arterial vessel wall, which might lead to recurrent thrombosis and distal embolism.In this study the intracranial vessel wall was evaluated in patients with acute ischemic stroke after IAT using high-resolution vessel wall MRI. Thirteen patients underwent both 3T and 7T pre- and postcontrast vessel wall MRI to detect contrast-enhancing lesions. Significantly more enhancing vessel wall lesions and concentric enhancing lesions ipsilateral to IAT were found compared to the contralateral side. The higher number of concentric enhancing lesions ipsilateral to the IAT may be related to the presence of the thrombus and the performed IAT.
Purpose
To evaluate the intracranial vessel wall with (ultra-)high resolution MR vessel wall imaging after intra-arterial treatment (IAT) in patients with acute ischemic stroke.Background
IAT improves outcome in patients with acute ischemic stroke and occlusion of a proximal artery in the anterior circulation1-5. There is some concern that IAT may damage the arterial vessel wall, which might lead to recurrent thrombosis and distal embolism6-9. A recent MRI vessel wall study found more vessel wall enhancement and vessel wall thickening in patients treated with IAT compared to a control group10. In the present study each patient was scanned with both 3T and 7T pre- and postcontrast intracranial vessel wall MR-imaging to detect contrast-enhancing arterial lesions after IAT.Methods
Study population
We included a consecutive series of 13 acute ischemic stroke patients treated
with IAT were included (7 males, mean age 65 years). Patients were included if
they were able to undergo both MRI examinations within three months after
symptom onset. The study was approved by the Institutional Review Board of our
hospital and all patients gave written consent.
Treatment
IAT was
performed by means of thrombosuction in 10 patients (69%) (Penumbra), with a stent-retriever
(Solitaire) in 2 patients (23%) and with both strategies in 1 patient (8%). IAT
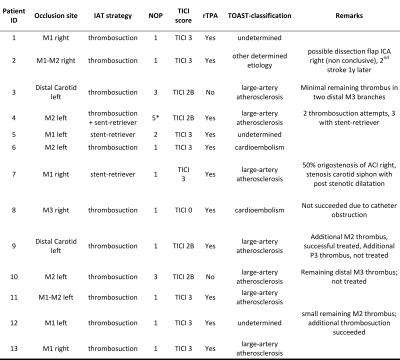
was technically successful in 12 of 13 patients. Treatment details are shown in
Table 1.
Imaging
All patients
were scanned with both 3T (Achieva, Philips Healthcare) and 7T (Philips
Healthcare) MRI. The scan protocol included a 3D T1-weighted Volumetric Isotropically
Reconstructed Turbo Spin-Echo Acquisition (VIRTA) sequence at 3T and a 3D T1-weighted
Magnetization Prepared Inversion Recovery Turbo Spin-Echo (MPIR-TSE) vessel
wall sequence at 7T11,12,13. All vessel wall scans were performed pre- and
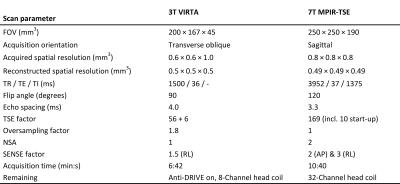
postcontrast. Table 2 shows the applied scan parameters. The mean time from
symptom onset to 3T MRI was 47 days (range 1–89 days) and to 7T 51 days (7–84 days).
Image assessment
3T and 7T images
were combined for assessment of vessel wall enhancement by an expert panel
consisting of a neuroradiologist and a neuro-interventional radiologist. Enhancement
was classified as a concentric or eccentric lesion. Analyzed arterial segments
included the distal internal carotid artery and the middle cerebral artery.
Findings were presented stratified for thrombectomy site, allowing for
comparison with the contralateral site as reference.
Statistical Analysis
A Wilcoxon
signed-rank test was used for comparison between the number and type of lesions
ipsilateral and contralateral to the thrombectomy site. Statistical
significance was set at p<0.05.
Results
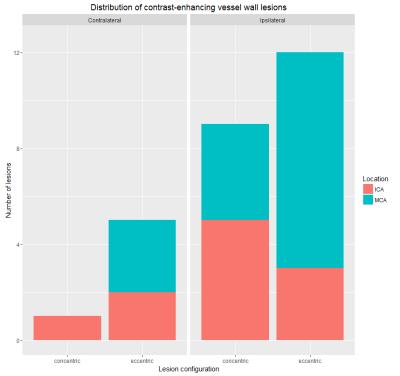
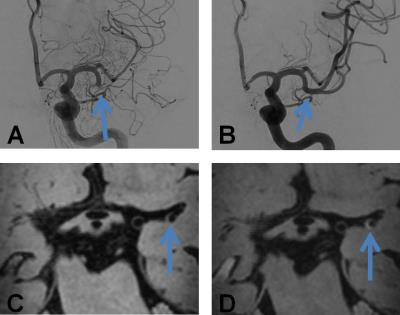
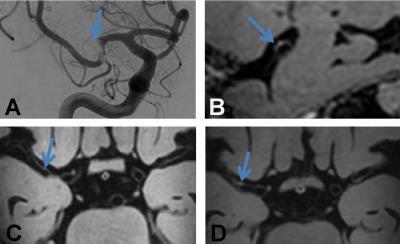
The total number of contrast-enhancing lesions, separated by side are shown in Figure 1. In total 27 contrast-enhancing lesions were found. There were more lesions on the ipsilateral side (n=21) compared to the contralateral side (n=6) (p<0.01). Specified for configuration, there were more concentric enhancing lesions ipsilateral (n=9) compared to contralateral (n=1) (p=0.01). There was no significant difference between the number of eccentric enhancing lesions on both sides (ipsilateral side n=12, and contralateral n=5 (ns; p=0.07)). The percentage of concentric enhancing lesions of the total number of lesions was higher on the ipsilateral side compared to the contralateral side (75% vs. 20%). Examples of a concentric and eccentric enhancing lesion at the thrombectomy site are shown in Figures 2 and 3, respectively.Discussion and Conclusion
In this study, significantly more enhancing vessel wall lesions and concentric enhancing lesions ipsilateral to IAT were found compared to the contralateral side. An explanation of these lesions may be the presence of the occlusive thrombus and consequently reactive (inflammatory) changes induced by this thrombus on the adjacent vessel wall segment14. Besides this direct effect, vessel wall changes may also be related to the thrombectomy procedure6-8. A possible explanation for the observed concentric wall enhancement is the arterial wall edema including endothelial damage which is described in histopathological studies performed after IAT6. Furthermore, pre-existing intracranial atherosclerotic lesions may be present in the revascularised segment. These type of lesions do have more often an eccentric configuration15. In general, the pathophysiological cause of vessel wall enhancement is possibly local inflammation of the vessel wall and an increased endothelial permeability due the mechanical forces and the presence of the thrombus and vasa vasorum10,14,16. A limitation of the study is that we used a relative long time interval between the thrombectomy and MR-imaging. In patients with a longer delay time short-term effects of the thrombus on the vessel wall may have disappeared. The advantage of including patients with a longer time interval is a better insight in the long-term effects of the thrombus and IAT of the arterial vessel wall.Acknowledgements
No acknowledgement found.References
1. Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. The New England journal of medicine 2015;372:11-20.
2. Saver JL, Jahan R, Levy EI, et al. SOLITAIRE with the intention for thrombectomy (SWIFT) trial: design of a randomized, controlled, multicenter study comparing the SOLITAIRE Flow Restoration device and the MERCI Retriever in acute ischaemic stroke. International journal of stroke : official journal of the International Stroke Society 2014;9:658-68.
3. Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet 2012;380:1231-40.
4. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. The New England journal of medicine 2015;372:1019-30.
5. Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. The New England journal of medicine 2015;372:1009-18.
6. Gory B, Bresson D, Kessler I, et al. Histopathologic evaluation of arterial wall response to 5 neurovascular mechanical thrombectomy devices in a swine model. AJNR American journal of neuroradiology 2013;34:2192-8.
7. Teng D, Pannell JS, Rennert RC, et al. Endothelial trauma from mechanical thrombectomy in acute stroke: in vitro live-cell platform with animal validation. Stroke; a journal of cerebral circulation 2015;46:1099-106.
8. Peschillo S, Diana F, Berge J, Missori P. A comparison of acute vascular damage caused by ADAPT versus a stent retriever device after thrombectomy in acute ischemic stroke: a histological and ultrastructural study in an animal model. Journal of neurointerventional surgery 2016.
9. Kurre W, Perez MA, Horvath D, Schmid E, Bazner H, Henkes H. Does mechanical thrombectomy in acute embolic stroke have long-term side effects on intracranial vessels? An angiographic follow-up study. Cardiovascular and interventional radiology 2013;36:629-36.
10. Power S, Matouk C, Casaubon LK, Silver FL, Krings T, Mikulis DJ. Vessel wall magnetic resonance imaging in acute ischemic stroke: effects of embolism and mechanical thrombectomy on the arterial wall. Stroke; a journal of cerebral circulation 2014;45.
11. Dieleman N, Yang W, van der Kolk AG, et al. Qualitative Evaluation of a High-Resolution 3D Multi-Sequence Intracranial Vessel Wall Protocol at 3 Tesla MRI. PloS one 2016;11:e0160781.
12. Qiao Y, Steinman DA, Qin Q, Etesami M, Schar M, Astor BC. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. Journal of magnetic resonance imaging : JMRI 2011;34.
13. van der Kolk AG, Hendrikse J, Brundel M, Biessels GJ, Smit EJ, Visser F. Multi-sequence whole-brain intracranial vessel wall imaging at 7.0 tesla. European radiology 2013;23:2996-3004.
14. Jin R, Liu L, Zhang S, Nanda A, Li G. Role of inflammation and its mediators in acute ischemic stroke. Journal of cardiovascular translational research 2013;6:834-51.
15. Swartz RH, Bhuta SS, Farb RI, Agid R, Willinsky RA, Terbrugge KG. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology 2009;72:627-34.
16. Portanova A, Hakakian N, Mikulis DJ, Virmani R, Abdalla WM, Wasserman BA. Intracranial vasa vasorum: insights and implications for imaging. Radiology 2013;267:667-79.
17. Fugate JE, Klunder AM, Kallmes DF. What is meant by "TICI"? AJNR American journal of neuroradiology 2013;34:1792-7.
18. Adams HP, Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke; a journal of cerebral circulation 1993;24:35-41.
Figures