0272
Evaluation of CSF Suppression Techniques for Intracranial Vessel Wall Imaging1Vanderbilt University Medical Center, Nashville, TN, United States, 2Radiology, University Medical Center Utrecht, Netherlands, 3Spinoza Center for Neuroimaging, Amsterdam, Netherlands, 4MR Clinical Science, Philips Healthcare Canada, Markham, Canada
Synopsis
This work compares vessel wall SNR and CSF suppression from multiple approaches to determine optimal imaging parameters for intracranial VWI at the clinically-available field strength of 3T. T1-weighted TSE acquisition using variable refocusing angle pulse-train and DANTE preparation provides for blood and CSF suppression while maintaining adequate vessel wall SNR. The use of a variable refocusing pulse train with sweep of 40-120° provides improved performance compared to a sweep of 50-120°. Variation of the DANTE flip angle showed that a flip angle of 8° provides good CSF suppression with minimal SNR loss compared to flip angles of 10 and 12°.
Introduction
Vessel wall imaging (VWI) of the intracranial vasculature has recently become available and may be of clinical use for differentiating types of vascular disease1 or evaluating atherosclerotic lesions that are not yet hemodynamically significant, as shown in other vessels2,3. To accurately assess the vessel wall, imaging must provide adequate suppression of intraluminal blood and surrounding cerebrospinal fluid (CSF) as well as maintain high signal-to-noise ratio (SNR) at the spatial resolution of the intracranial vessel walls (0.2-0.6 mm). Several methods have been implemented to null blood and CSF signal including variable flip angle turbo-spin-echo readouts4,5, inversion recovery preparations6, post-readout anti-DRIVE modules7 and DANTE preparations8–10. However, while a broad consensus on the relevance of intracranial VWI exists11, a consensus on optimal clinical sequence parameters is currently unavailable. Therefore, the goal of this work is to compare vessel wall SNR and CSF suppression from the above candidate approaches to determine optimal imaging parameters for intracranial VWI at the clinically-available field strength of 3T.Materials and Methods
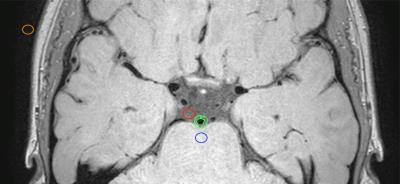
Imaging: Scans were performed on a 3T whole body scanner (Philips Medical Systems, Best, The Netherlands) using body coil transmission and a 32 channel receiver head coil. The base T1-weighted 3D-TSE acquisition was axial orientation with TSE factor =56, TR/TE=1500/33ms, field-of-view=200x166x45 mm3, spatial resolution=0.5x0.5x1.0mm3, and duration=4min42s. Blood and CSF suppression methods tested were motivated by Bloch equation simulations and prior experimental reports and included: (i) T1-weighted TSE with variable refocusing angle pulse-train using sweep=40-120° and sweep=50-120° (ii) T1-weighted TSE with DANTE preparation8,9 (repetitions=300, interval=1.1 ms, gradient strength in three directions=22.5mT/m) using 8, 10, and 12º flip angles, (iii) T2-weighted TSE readout (TSE factor=15, TR/TE=4000/83ms, field-of-view=200x166x45 mm3, spatial resolution=0.5x0.4x1.0 mm3, duration=5min12s), and (iv) T1-weighted inversion recovery TSE readout6 (TSE factor=25, TR/TI/TE=800/400/36ms, field-of-view=250x250x170 mm3, spatial resolution 0.5x0.5x1.0 mm3, duration 8min48s). Subjects: Ten healthy adults (mean age 39±17 years, range 22-60 years) were imaged with each of the above sequences. Inclusion criterion was age 18-65 years. Exclusion criteria included prior stroke, TIA, heart attack, diabetes, smoking, and BMI > 40. Analysis: Analysis was performed using Osirix (Pixmeo, Bernex, Switzerland). SNR of the CSF (measured in the prepontine cistern), parenchyma (central pons), and vessel wall (basilar artery) were calculated as the ratio of the mean signal to standard deviation of thermal noise. ROIs with areas approximately 10 mm2 were used for the background, CSF, and pons (Figure 1). Vessel wall ROIs were hand drawn. The ROIs were drawn on acquisition (i) using sweep=40-120°and copied to the others series in that exam. Mean and standard deviations of the signal and SNR were calculated for each CSF suppression technique. A Wilcoxon signed-rank test was used to evaluate measurements from different scan sequences applied in the same subjects.Results
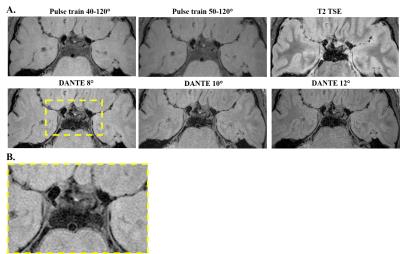
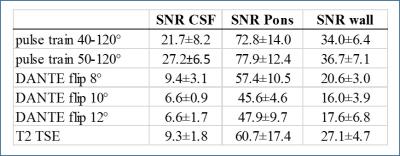
Representative images from one subject are shown in Figure 2. Table 1 summarizes the SNR results. The inversion recovery acquisition was of insufficient quality to make vessel wall measurements and was therefore not included in analysis. CSF suppression was improved (one-sided p=0.034) when using sweep=40-120° compared to sweep=45-120°. CSF suppression was significantly improved (two-sided p<0.01) with the addition of the DANTE preparation with approximately 3x reduction in mean CSF signal between variants (i) and (ii). However, the DANTE preparation also resulted in significantly reduced (p<0.01) vessel wall signal of approximately 2x between variants (i) and (ii). With the DANTE scans, CSF suppression improved (two-sided p<0.01) from DANTE flip angle 8° to DANTE 10 and 12° but not between DANTE 10 and 12°. Vessel wall signal was also reduced going from DANTE flip angle 8° to DANTE 12° (two sided p<0.01) but not from DANTE 10 to 12°. The T2-weighted TSE readout (iii) provided similar CSF suppression and vessel wall signal as the DANTE preparation with 8° flip angle.Conclusions
The variable flip angle T1-weighted TSE acquisition provides blood and CSF suppression that allows for visualization of the vessel wall in healthy subjects, and the CSF suppression is improved with sweep=40-120° versus sweep=50-120°. Addition of the DANTE preparation results in further reduction in CSF signal, eradicating CSF signal that may obscure or simulate vessel wall lesions. However, the improved CSF suppression comes at the cost of a decrease in the vessel wall signal, which is likely in part due to the pulsation of the vessel wall, affected by the motion sensitive DANTE pulse10. Addition of the DANTE preparation with a flip angle of 8° minimizes SNR losses. Based on these comparisons, we recommend a 0.5 mm variable refocusing angle (40-120°) TSE acquisition and DANTE preparation with flip angle 8° in patients with intracranial stenosis.Acknowledgements
No acknowledgement found.References
1. Mossa-Basha, M. et al. Multicontrast high-resolution vessel wall magnetic resonance imaging and its value in differentiating intracranial vasculopathic processes. Stroke J. Cereb. Circ. 46, 1567–1573 (2015).
2. Babiarz, L. S., Astor, B., Mohamed, M. A. & Wasserman, B. A. Comparison of gadolinium-enhanced cardiovascular magnetic resonance angiography with high-resolution black blood cardiovascular magnetic resonance for assessing carotid artery stenosis. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 9, 63–70 (2007).
3. Glagov, S., Weisenberg, E., Zarins, C. K., Stankunavicius, R. & Kolettis, G. J. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 316, 1371–1375 (1987).
4. Qiao, Y. et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. J. Magn. Reson. Imaging Jmri 34, 22–30 (2011).
5. Hennig, J., Weigel, M. & Scheffler, K. Multiecho sequences with variable refocusing flip angles: optimization of signal behavior using smooth transitions between pseudo steady states (TRAPS). Magn. Reson. Med. 49, 527–535 (2003).
6. Kolk, A. G. van der et al. Intracranial Vessel Wall Imaging at 7.0-T MRI. Stroke 42, 2478–2484 (2011).
7. Harteveld, A. A. et al. High-resolution intracranial vessel wall MRI in an elderly asymptomatic population: comparison of 3T and 7T. Eur. Radiol. (2016). doi:10.1007/s00330-016-4483-3
8. Li, L., Miller, K. L. & Jezzard, P. DANTE-prepared pulse trains: a novel approach to motion-sensitized and motion-suppressed quantitative magnetic resonance imaging. Magn. Reson. Med. 68, 1423–1438 (2012).
9. Wang, J. et al. Joint blood and cerebrospinal fluid suppression for intracranial vessel wall MRI. Magn. Reson. Med. 75, 831–838 (2016).
10. Viessmann, O., Li, L., Benjamin, P. & Jezzard, P. T2-Weighted intracranial vessel wall imaging at 7 Tesla using a DANTE-prepared variable flip angle turbo spin echo readout (DANTE-SPACE). Magn. Reson. Med. (2016). doi:10.1002/mrm.26152
11. Mandell, D. M. et al. Intracranial Vessel Wall MRI: Principles and Expert Consensus Recommendations of the American Society of Neuroradiology. Am. J. Neuroradiol. (2016). doi:10.3174/ajnr.A4893
Figures