0271
In-vivo Detection of Remote Neurodegeneration within Thalamic Nuclei after Stroke Using Iron Quantification with R2* Mapping1Neuroradiology, Univ. Lille, CHU Lille, Lille, France, 2Neuroimagerie diagnostique et thérapeutique, Université de Bordeaux, CHU de Bordeaux, France, 3Richard M. Lucas Center for Imaging Radiology Department, Stanford University, United States, 4Unité neurovasculaire, Université de Bordeaux, CHU de Bordeaux, France
Synopsis
In stroke patients, remote thalamic alterations including iron deposition have been reported and attributed to the disruption of cortico-thalamic projections. Nevertheless, secondary thalamic degeneration has never been quantified so far in humans at the nucleus scale and its clinical impact is unknown. By using R2* mapping, we demonstrated (i) that iron accumulates with a focal distribution especially within the medio-dorsal nucleus and the pulvinar, (ii) that such focal thalamic iron accumulation is strongly linked to the initial stroke location, consistent with the known connectivity between thalamic nuclei and cortico-subcortical areas and (iii) is significantly impacting specific cognitive and emotional functions.
Introduction
In stroke patients, remote thalamic alterations have been reported and attributed to the disruption of cortico-thalamic projections.1-4 However, the thalamus is composed of several nuclei,5 each nucleus having its own connectivity pattern6,7 and driving specific functions.8 Individual thalamic nuclei should be differentially affected by secondary degeneration depending on the initial stroke location. Nevertheless, secondary thalamic degeneration has never been quantified so far in humans at the nucleus scale. The aim of the study was (i) to demonstrate regional accumulation of iron as quantified by R2* in specific thalamic nuclei in stroke patients and (ii) to demonstrate that it represents secondary damages in direct relation with the location of the initial stroke and (iii) that it is responsible for specific cognitive and emotional impairment.Methods
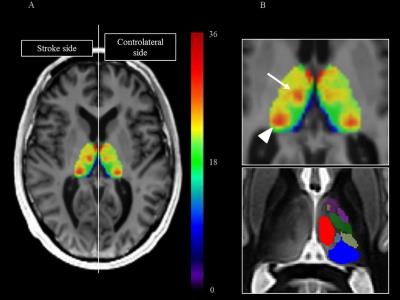
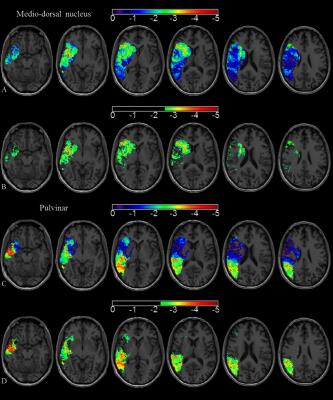
One hundred and seventy-two patients with a stroke sparing the thalamus were prospectively assessed at 24-72 hours and 1 year with MRI on a 3T scanner. The MR protocol included a 2D multi-echo fast gradient-echo (FGRE) sequence with 8 echo times (4.3, 8.7, 13.0, 17.4, 21.7, 26.1, 30.5 and 34.8ms) allowing R2* mapping.9 R2* values were measured within the entire thalamus and within the medio-dorsal nucleus and the pulvinar using 3D-atlas-driven regions-of-interest.10,11 First, an asymmetry index (AI) was used to compare R2* values within the thalamus ipsilateral versus contralateral to stroke. The spatial distribution of iron accumulation was analyzed on an average R2* map.12 Then, to demonstrate the relationship between focal iron accumulation in specific nuclei and initial stroke location, AI within the medio-dorsal nucleus and the pulvinar were compared against the involvement or not of the different cortical locations and deep nuclei in univariate and multivariate analyses. We also built maps of stroke location responsible for specific iron accumulation in the medio-dorsal or in the pulvinar using an adaptation of the voxel-based symptom-lesion mapping (VLSM) method.13,14 Finally, we calculated with multivariate regression models the associations between iron accumulation measured within the entire thalamus or within specific thalamus nuclei and functional outcome evaluated by the modified Rankin scale at 1 year, cognitive outcome evaluated by the Montreal Cognitive Assessment (MoCA) score at 1 year15 and emotional outcome evaluated by the Hospital Anxiety and Depression scale at 1 year.16Results
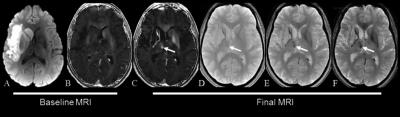
R2* in the entire thalamus ipsilateral to stroke was not modified at 24-72 hours but showed heterogeneous increase at 1 year especially within the medio-dorsal nucleus and the pulvinar (Fig 1 and 2). High AI within the medio-dorsal nucleus was significantly associated with anterior stroke location (frontal p=0.05, temporal p=0.02, lenticular p=0.01) while high AI within the pulvinar was significantly associated with posterior stroke location (parietal p=0.046, temporal p<.001) independently of age, gender and stroke volume, which was confirmed on VLSM maps (Fig 3). A high AI in the entire thalamus at 1 year was independently associated with a poor cognitive outcome defined by the MoCA score in patients with left-hemispheric stroke (adjusted OR=6.48, 95%CI=1.27-32.9, p=0.02), with post-stroke anxiety (adjusted OR= 2.23, 95%CI=1.04-4.79, p=0.04) and post-stroke depression (adjusted OR= 2.77, 95%CI, 1.14-6.72, p=0.02). At the nucleus level, a high AI in the pulvinar was associated with a specific alteration of the visuospatial MoCA subscore (OR=2.17, 95%CI =0.99-4.63, p=0.055) and with post-stroke anxiety in patients with right-hemispheric stroke (OR=3.36, 95%CI=1.16-9.69, p=0.03).A high AI in the medio-dorsal nucleus tended to be associated with a specific alteration of the recall MoCA subscore in patients with left-hemispheric stroke (OR=3.60, 95%CI=0.68-19.2, p=0.13) and with post-stroke depression in patients with right-hemispheric stroke (OR=5.57, 95%CI=1.35-22.9, p=0.02).Discussion
R2* mapping is a suitable method to demonstrate and quantify secondary remote neurodegeneration after stroke at the scale of the thalamic nucleus. Iron accumulates in the thalamus ipsilateral to stroke after a delay, with a focal distribution especially within the medio-dorsal nucleus and the pulvinar, which is strongly linked to the initial stroke location, consistently with the known connectivity pattern between each thalamic nucleus and cortico-subcortical areas. Such remote thalamic neurodegenerative lesions were independently associated with a poor cognitive and emotional outcome.Conclusion
Remote thalamic neurodegeneration can be quantified in vivo with R2* mapping in stroke patients. The potential impact of these secondary lesions on cognitive and emotional outcome needs to be confirmed in larger longitudinal studies. This secondary neurodegenerative stream is a potential therapeutic target for neuronal protection after ischemic stroke.Acknowledgements
No acknowledgement found.References
1. Matthews MA. Death of the central neuron: an electron microscopic study of thalamic retrograde degeneration following cortical ablation. J Neurocytol. 1973;2(3):265-288.
2. Iizuka H, Sakatani K, Young W. Neural damage in the rat thalamus after cortical infarcts. Stroke. 1990;21(5):790-794.
3. Tamura A, Tahira Y, Nagashima H, et al. Thalamic atrophy following cerebral infarction in the territory of the middle cerebral artery. Stroke. 1991;22(5):615-618.
4. Nakane M, Tamura A, Nagaoka T, et al. MR detection of secondary changes remote from ischemia: preliminary observations after occlusion of the middle cerebral artery in rats. AJNR Am J Neuroradiol. 1997;18(5):945-950.
5. Morel A, Magnin M, Jeanmonod D. Multiarchitectonic and stereotactic atlas of the human thalamus. J Comp Neurol. 1997;387(4):588-630.
6. Behrens TE, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6(7):750-757.
7. Johansen-Berg H, Behrens TE, Sillery E, et al. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb Cortex. 2005;15(1):31-39.
8. Saalmann YB, Kastner S. The cognitive thalamus. Front Syst Neurosci. 2015;9(39):39.
9. Langkammer C, Krebs N, Goessler W, et al. Quantitative MR imaging of brain iron: a postmortem validation study. Radiology. 2010;257(2):455-462.
10. Tourdias T, Saranathan M, Levesque IR, et al. Visualization of intra-thalamic nuclei with optimized white-matter-nulled MPRAGE at 7T. Neuroimage. 2014;84:534-545.
11. Su JT, Saranathan M, Rutt BK. THOMAS: Thalamus Optimized Multi-Atlas Segmentation. Proc Intl Soc Mag Reson Med. 2015;23:6231.
12. Peran P, Hagberg G, Luccichenti G, et al. Voxel-based analysis of R2* maps in the healthy human brain. J Magn Reson Imaging. 2007;26(6):1413-1420.
13. Bates E, Wilson SM, Saygin AP, et al. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6(5):448-450.
14. Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19(7):1081-1088.
15. Lees R, Selvarajah J, Fenton C, et al. Test accuracy of cognitive screening tests for diagnosis of dementia and multidomain cognitive impairment in stroke. Stroke. 2014;45(10):3008-3018.
16. Prisnie JC, Fiest KM, Coutts SB, et al. Validating screening tools for depression in stroke and transient ischemic attack patients. Int J Psychiatry Med. 2016;51(3):262-277.
Figures