0269
Estimation of microstructure measures in stroke subjects with a rapid DSI acquisition1Radiology and Imaging Sciences, University of Utah, Salt lake city, UT, United States, 2Bioengineering, University of Utah, Salt lake city, UT, United States, 3Neurology, University of Utah, Salt Lake City, UT, United States, 4Occupational Therapy, University of Utah, Salt Lake City, UT, United States
Synopsis
Diffusion spectrum imaging (DSI) is a promising tool for estimation of white-matter fiber structure. DSI also allows for model-based estimation of several microstructure measures. However, the long data acquisition time associated with DSI limits its application in stroke patients. Here we combine a simultaneous multi-slice acquisition with an undersampled q-space acquisition and dictionary reconstruction to accelerate DSI. The two complementary acceleration schemes allow for a rapid 5.5 minute DSI acquisition in stroke subjects. We used generalized fractional anisotropy and microstructure measures computed from the NODDI model to evaluate the rapid DSI framework in stroke patients.
Purpose
To enable estimation of advanced microstructure measures in stroke subjects using short diffusion spectrum imaging (DSI) scans. DSI provides a model-free approach for estimation of white-matter fiber structure[1]. The acquisition also allows for model-based estimation of several microstructure measures including generalized fractional anisotropy (GFA)[2] as well as orientation dispersion index (ODI), and restricted diffusion index(RDI) and cerebro-spinal fluid fraction(CSF) measures from the neurite-orientation dispersion and density index (NODDI) model[3, 4]. However, the long scan times typically required for DSI limits their use and limits our understanding of the utility of such higher order diffusion parameters in patients with stroke. Here we use a simultaneous multi-slice (SMS) slice acceleration technique[5, 6] in conjunction with q-space undersampling and a fast dictionary reconstruction[7] to acquire DSI data in under 6 minutes in stroke subjects.Methods
SMS acquisition:Fully sampled DSI data (203 directions, 12 minutes) was acquired in 6 subjects 1-5 months post-stroke. We used a blipped CAIPI SMS acquisition[5, 6] with a slice acceleration factor of three, no in-plane k-space undersampling, and 203 diffusion encoding directions with a maximum b=4000 [8]. The data was acquired on a Siemens 3T Verio scanner using a 32 channel head coil. The scan parameters were TR=3500 msec, TE=114 msec, FOV=250 mm2, pixel size=2 mm2, slice thickness=2.1 mm, number of slices=51. A split-slice GRAPPA reconstruction was used to reconstruct the SMS data[9].
q-space undersampling:In order to further accelerate the data acquisition, we studied undersampling the q-space of the above 203 direction SMS acquisition and then reconstructing the diffusion probability density functions (PDFs) using a dictionary-based method[7]. The reconstruction used a trained dictionary of PDFs with principal component analysis. Estimates of new PDFs from undersampled q-space data were done in a non-iterative fashion. That is, a new PDF was represented by a first few principal components from the training dictionary while preserving data fidelity[7]. Training dictionary of PDFs (on a 17x17x17 grid) was built using fully sampled SMS q-space data from three stroke subjects.
Retrospective undersampling:Fully sampled q-space data from a stroke subject, not used in building the dictionary, was retrospectively undersampled in a variable density random fashion[10]. Data was undersampled by different amounts: R=1.45,R=2.31,R=3.12 and R=4.23 (R=reduction factor). Discarded diffusion directions were estimated by Fourier transforming the reconstructed PDFs. Microstructure measures were computed using all of the estimated diffusion directions. We used GFA maps[11] and RDI, ODI, CSF maps computed from the NODDI model[3]. Evaluation of the reconstructions was done by comparing the maps from fully sampled and undersampled q-space acquisitions.
Prospective undersampling:Prospectively undersampled q-space data combined with SMS acquisitions was acquired in two subjects (different from the subjects used to build the dictionary) with a q-space undersampling of R=2.31. All of the other scan parameters remained the same as the fully sampled q-space acquisitions. The scan time for the undersampled acquisitions was 5.5 minutes. Fully sampled q-space was also acquired in these two subjects for comparison. Microstructure measures were computed in regions of interest.
Results
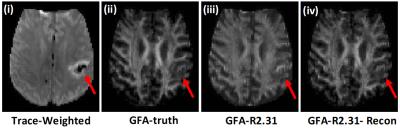
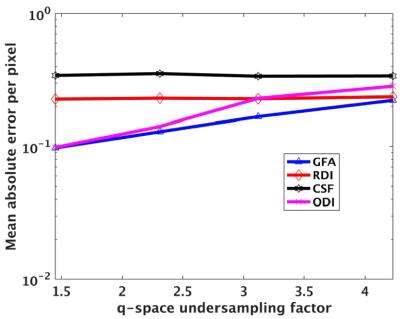
Figure 1 shows the results from retrospective undersampling of full q-space data. Figure 1a shows GFA maps from fully sampled and undersampled q-space by a factor of R=2.31. The GFA map quality is significantly improved by using the dictionary based reconstruction and matches closely with the ground truth GFA image. Figure 1b shows plots of normalized root mean squared errors (NRMSE) for different undersampling factors for GFA as well as the ODI, RDI, CSF maps computed using the NODDI model. Errors increase gradually with increasing undersampling factors for GFA and RDI measures while not changing significantly for ODI and CSF measures.
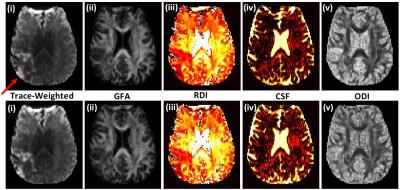
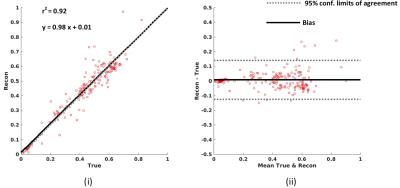
Figure 2 shows the results from the prospective 5.5 minute (R=2.31) DSI acquisitions. Figure 2a shows trace weighted images, GFA, and NODDI maps from the 12 minute and 5.5 minute DSI acquisitions. Regions of interest encompassing stroke lesions in all of the acquired slices were drawn manually and mean microstructure measure in the ROI was computed. Figure 2b shows the correlation and Bland-Altman plots for all of the mean microstructure measures for two subjects between the fully sampled and undersampled acquisitions. Average reconstruction time for a slice was ~2.2 min on a 16 core Linux machine in Matlab.
Conclusion
SMS acquisition with q-space undersampling and fast dictionary based reconstruction framework is promising for rapid DSI acquisitions in stroke subjects. The framework allows for the estimation of advanced microstructure measures with little or no loss in quality. Future work involves determining the potential of these advanced microstructure measures for improved understanding of stoke and possible predictions of recovery using the rapid data acquisition framework.Acknowledgements
This work is supported by R01NS083761.References
[1] V.J. Wedeen, P. Hagmann, W.-Y.I. Tseng, T.G. Reese, R.M. Weisskoff, Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging, Magnetic Resonance in Medicine, 54 (2005) 1377-1386.
[2] D.S. Tuch, Q-ball imaging, Magnetic Resonance in Medicine, 52 (2004) 1358-1372.
[3] H. Zhang, T. Schneider, C.A. Wheeler-Kingshott, D.C. Alexander, NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain, NeuroImage, 61 (2012) 1000-1016.
[4] G. Adluru, Y. Gur, J.S. Anderson, L.G. Richards, N. Adluru, E.V.R. DiBella, Assessment of white matter microstructure in stroke patients using NODDI, in: Engineering in Medicine and Biology Society (EMBC), 2014 36th Annual International Conference of the IEEE, 2014, pp. 742-745.
[5] S. Moeller, E. Yacoub, C.A. Olman, E. Auerbach, J. Strupp, N. Harel, K. Ugurbil, Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI, Magnetic Resonance in Medicine, 63 (2010) 1144-1153.
[6] K. Setsompop, B.A. Gagoski, J.R. Polimeni, T. Witzel, V.J. Wedeen, L.L. Wald, Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty, Magnetic Resonance in Medicine, 67 (2012) 1210-1224.
[7] B. Bilgic, I. Chatnuntawech, A.P. Fan, K. Setsompop, S.F. Cauley, L.L. Wald, E. Adalsteinsson, Fast image reconstruction with L2-regularization, Journal of Magnetic Resonance Imaging, 40 (2014) 181-191.
[8] L.-W. Kuo, J.-H. Chen, V.J. Wedeen, W.-Y.I. Tseng, Optimization of diffusion spectrum imaging and q-ball imaging on clinical MRI system, NeuroImage, 41 (2008) 7-18.
[9] S.F. Cauley, J.R. Polimeni, H. Bhat, L.L. Wald, K. Setsompop, Interslice leakage artifact reduction technique for simultaneous multislice acquisitions, Magnetic Resonance in Medicine, 72 (2014) 93-102.
[10] M. Lustig, D. Donoho, J.M. Pauly, Sparse MRI: The application of compressed sensing for rapid MR imaging, Magnetic Resonance in Medicine, 58 (2007) 1182-1195.
[11] E. Garyfallidis, M. Brett, B. Amirbekian, A. Rokem, S. Van Der Walt, M. Descoteaux, I. Nimmo-Smith, Dipy, a library for the analysis of diffusion MRI data, Frontiers in Neuroinformatics, 8 (2014).
[12] J. Chong, D. Lu, F. Aragao, M.B. Singer, W.J. Schonewille, A. Silvers, S. Tuhrim, S.W. Atlas, Diffusion-weighted MR of acute cerebral infarction: comparison of data processing methods, American Journal of Neuroradiology, 19 (1998) 1733-1739.
[13] M.-C. Chou, W.-S. Tzeng, H.-W. Chung, C.-Y. Wang, H.-S. Liu, C.-J. Juan, C.-P. Lo, C.-J. Hsueh, C.-Y. Chen, T2-enhanced tensor diffusion trace-weighted image in the detection of hyper-acute cerebral infarction: Comparison with isotropic diffusion-weighted image, European Journal of Radiology, 74 (2010) e89-e94.
Figures