0264
A ranking of pipelines for optimal co-registration of anatomical and diffusion weighted images of the cervical spinal cord1UCL Institute of Neurology, Queen Square MS Centre, University College London, London, United Kingdom, 2Translational Imaging Group, Centre for Medical Image Computing, Department of Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 3Department of Brain and Behavioural Sciences, University of Pavia, Pavia, Italy, 4Brain MRI 3T Mondino Research Center, C. Mondino National Neurological Institute, Pavia, Italy
Synopsis
We conduct the first systematic evaluation of the performance of three widely used registration software toolkits (FLIRT from FSL, NiftyReg and ANTs employing Spinal Cord Toolbox) in an effort to outline a method for reliable co-registration between anatomical and quantitative (EPI-based) spinal cord MRI. We generate a diverse set of registration pipelines and rank them according to quality of co-registration metrics. We find that ANTs and NiftyReg outperform FLIRT, and we report heterogeneity of specifications for optimal co-registration among software toolkits.
Purpose
Quantitative
magnetic resonance imaging (q-MRI) is a promising tool for the
investigation of microstructural tissue properties of the spinal
cord. To date, there is a notable lack of standardised processing
pipelines for co-registering q-MRI images to high-resolution
anatomical reference scans. Here, we aim to find an optimal strategy
for reliable co-registration between quantitative and anatomical MRI
of the spinal cord using publicly available toolkits, with a focus on
EPI for diffusion-weighted (DW) acquisitions.Methods
Subjects and acquisition:
We retrospectively analysed scans of the cervical spine from 28 healthy subjects from a previous study.
Acquisition protocol: 1) DWI: reduced field-of-view (FOV) (ZOOM) = 64x48mm2, resolution = 1x1x5mm3, slices=10, cardiac gating (triggering delay 150ms), TR=12RR beats, TE=52ms, b=1000s/mm2 (30 directions, 3 b=0); 2) anatomical 3D fast-field-echo (FFE): FOV = 180x240mm2, resolution = 0.5x0.5x5mm3, slices=10 (matched to diffusion), TR=23ms, TE=5ms, flip angle=7°.
Pre-processing:
Motion was corrected on the DW data with slice-wise linear registration1. Whole-cord semi-automated segmentation was performed on both DW and FFE data2, while the grey matter was segmented manually on each set of original images3. The FFE images were resampled to the DW resolution, and the spinal cord was straightened on both DW and FFE data sets.
Co-registration :
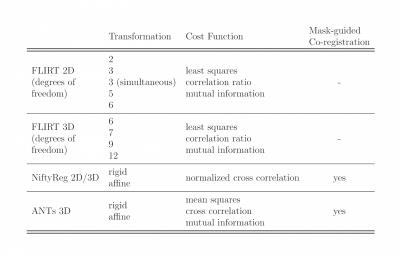
The co-registration was performed warping the mean b=0 image to the resampled FFE. We used flirt4,5 from FSL6, reg_aladin from NiftyReg7,8, and ANTs9 (using Spinal Cord Toolbox sct_register_multimodal10) to implement a number of co-registration pipelines, varying the registration parameters according to available options (Figure 1), with each combination of parameters representing a unique pipeline. In summary, co-registrations were i) 2D/3D, ii) rigid/affine, iii) characterised by different degrees of freedom (2 to 12), iv) cost functions and iv) inclusion of binary masks or not (depending on the options of the software used). We generated 27 pipelines with FLIRT, 16 with NiftyReg, and 12 with ANTs (total of 55).
Evaluation of co-registration:
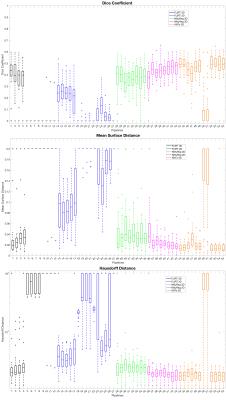
We evaluated the quality of co-registration studying three commonly used metrics: the Dice coefficient (DC), mean surface distance (MSD), and Hausdorff distance (HD)11. DC, MSD and HD were studied at two levels: using the co-registered whole-cord masks and using the co-registered grey matter masks. Co-registration pipelines were ranked according to DC, MSD and HD indices in turn.
Results
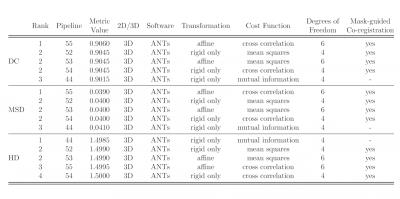
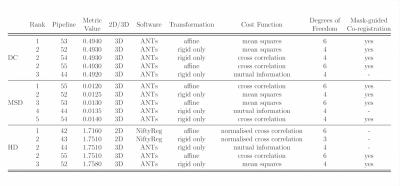
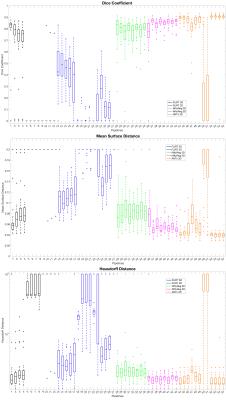
Figures 2 and 3 show the top 5 scoring pipelines at whole-cord and grey matter level. The ranking remains fairly consistent across metrics, with ANTs ranking best at whole-cord level and ANTs and NiftyReg at grey matter level. Figure 4 shows the distribution of DC, MSD and HD values across the 28 subjects at whole-cord level. In general, ANTs performs slightly better than NiftyReg on average, and they both outperform FLIRT for whole-cord registrations (Fig. 4). Figure 5 shows similar information as figure 4, but at grey matter level. The overall trends at grey matter level were similar as those at whole-cord level, though the quality of co-registration was lower (lower DC and higher MSD and HD). Finally, we also report that the best co-registration options (i.e. type of transformation, cost function and inclusion/non-inclusion of masks) varied among the best pipelines provided by each software toolkit considered in the study.Discussion and Conclusion
In this work, we have systematically compared for the first time the performance of three common software toolkits to identify an optimal approach for co-registration of quantitative (DW EPI-based) and anatomical images of the spinal cord. Specifically, we ranked 55 co-registration pipelines at whole-cord and grey matter levels. Our ranking shows that, using these specific datasets, the biggest impact on the quality of co-registration is the choice of software toolkit. While similar combinations of co-registration parameters provide optimal co-registration for one toolkit, they instead do not provide the best co-registration for the others. This suggests that the choice of the toolkit itself could have a big impact on the co-registration outcome, rather than the choice of the parameters.
We conclude that ANTs (Spinal Cord Toolbox) and NiftyReg are valid co-registration toolkits for the type of data considered here, and that the optimal combination of co-registration parameters needs ad hoc tuning depending on the software employed for analysis. In the future, more sophisticated co-registration pipelines (e.g. including non-linear registration), additional q-MRI/anatomical modalities and the generality of the results independently of the input/target data (i.e. readout and coverage) will be studied.
Acknowledgements
UK Multiple Sclerosis Society; Horizon 2020-EU.3.1 CDS-QUAMRI grant (ref.: 634541); UCL-UCLH Biomedical Research Centre; National Institute of Health Biomedical Research Centre UCLH/UCL High Impact Initiative (BW.mn.BRC10269); International Spinal Research Trust (ISRT); Engineering and Physical Sciences Research Council (EPSRC EP/H046410/1, EP/J020990/1, EP/K005278); MRC (MR/J01107X/1); Wings for Life; Craig H. Neilsen Foundation.References
[1] Grussu, F. et al. Neurite orientation dispersion and density imaging of the healthy cervical spinal cord in vivo. NeuroImage 2015; 111: 590-601.
[2] Horsfield, M.A. et al. Rapid semi-automatic segmentation of the spinal cord from magnetic resonance images: Application in multiple sclerosis. NeuroImage 2010; 50(2): 446-455.
[3] Kearney, H. et al. Spinal cord grey matter abnormalities are associated with secondary progression and physical disability in multiple sclerosis. Journal of Neurology, Neurosurgery & Psychiatry 2015; 86(6): 608-614.
[4] Jenkinson, M. et al. A global optimisation method for robust affine registration of brain images. Medical Image Analysis 2001; 5(2):143-156.
[5] Jenkinson, M. et al. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage 2002; 17(2):825-841.
[6] Jenkinson, M. et al. Fsl. NeuroImage 2012; 62(2): 782-90.
[7] Modat, M. et al. Global image registration using a symmetric block-matching approach. Journal of Medical Imaging 2014; 1(2): 024003.
[8] Modat M. et al. Fast free-form deformation using graphics processing units. Computer methods and programs in biomedicine 2010; 98(3): 278-84.
[9] Avants, Brian B. et al. The Insight ToolKit Image Registration Framework. Frontiers in Neuroinformatics 2014; 8:44.
[10] De Leener, B. et al. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. NeuroImage 2016; http://dx.doi.org/10.1016/j.neuroimage.2016.10.009.
[11] Klein, A. et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage 2009; 46(3): 786-802.
Figures