0250
Functional connectivity is globally altered by schizophrenia-linked genes1Radiology and Biomedical Imaging, Yale University, New Haven, CT, United States, 2Magnetic Resonance Research Center (MRRC), Yale University, New Haven, CT, United States, 3Neuroscience, Tufts University School of Medicine, Boston, MA, United States, 4Quantitative Neuroscience with Magnetic Resonance (QNMR) Core Center, Yale University, New Haven, CT, United States, 5Cellular Neuroscience, Neurodegeneration, and Repair Program, and Departments of Neurology and Neurobiology, Yale University School of Medicine, New Haven, CT, United States, 6Biomedical Engineering, Yale University, New Haven, CT, United States
Synopsis
Resting state fMRI shows increased global synchrony in schizophrenia at rest, but mechanisms remain speculative. We tested mice with knockout of SynCAM1 (related to synaptic organization), LRRTM1 (related to schizophrenia symptoms) and both genes using whisker-stimulation and resting-state fMRI. SynCAM1 was linked to stronger whisker barrel activation and to greater functional connectivity. However, this was lost if global signal regression was performed. Global signal amplitude was significantly higher in SynCAM1 knockout mice, and amplified by the additional knockout of LRRTM1. We hypothesize this is due to disrupted synaptic connections by SynCAM1 knockout, which are partially protected when LRRTM1 is present.
PURPOSE
Resting state fMRI of patients with schizophrenia indicates increased global synchrony at rest1. However, the global synchrony observed in functional imaging studies is poorly understood in terms of a mechanism2. To study potential mechanisms, resting state fMRI was used to scan mice with knockout of two genes: LRRTM1, which produces a transmembrane protein and is linked to schizophrenia symptoms3, and SynCAM1, which produces a protein used in organizing synapses4,5.METHODS
Imaging was performed using a 9.4T small-bore Bruker Biospec system. Four groups of mice in a C57BL/6 background were imaged, wild-type (WT, n=4), SynCAM1 knockout (SCKO, N=4), LRRTM1 knockout (n=6, LRKO) and double knockout of both SynCAM1 and LRRTM1 (n=4, DKO). Mice were anesthetized with urethane (1.5g/kg B.W). Two sets of functional imaging were collected with similar parameters (25.6x12.8mm FOV, 64x32 matrix, 8 slices, 1s TR, 13ms TE). First, four runs of fMRI during stimulation of the left whiskers were collected at 1Hz for 120s (30s baseline / 30s stimulation / 60s baseline), then one run of resting state fMRI was recorded for 2048s at 0.5Hz (1s TR but reference scan every image). Corresponding anatomical images were then collected using a spin echo sequence.
Functional data were slice-timing corrected, motion corrected, spatially blurred, and within-slice up-sampled to match anatomical image sizes prior to data analysis. Data were filtered to 0.01 to 0.03Hz6 and then data were analyzed both without, and with regression of the mean signal from the whole-brain, or “global signal regression”7. The mean signals in the left S1BF and the right S1BF were correlated. 512s segments were kept if they showed significant positive correlation (p ≤ 0.05, Z ≥ 1.64). This excluded one entire LRKO and one entire DKO mouse.
ANOVA was used to test for significance with terms for knockout of SynCAM1 gene (SCKO and DKO), knockout of LRRTM1 gene (LRKO and DKO) and interaction.
RESULTS
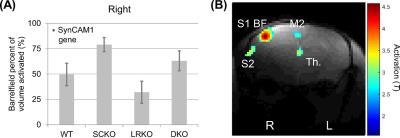
A significant effect of the SynCAM1 gene was seen in increasing the response to whisker activation (p=0.023 SynCAM1, p=0.097 LRKO, p=0.93 interaction) (Figure 1).
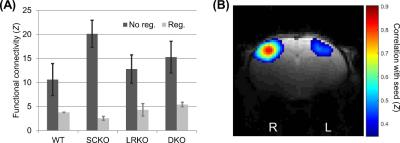
There were no significant genotypic differences in S1BF functional connectivity either prior to (p=0.083 SynCAM1, p=0.68 LRRTM1, p=0.29 interaction) or after (p=0.96 SynCAM1, p=0.23 LRRTM1, p=0.39 interaction) global signal regression (Figure 2). However, prior to global signal regression the profile of results was similar to activation but not following.
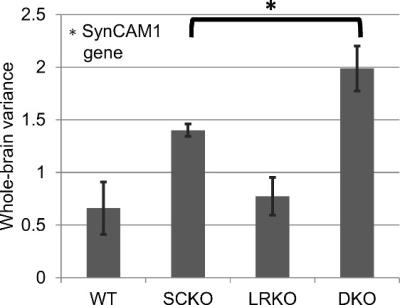
Following previous work1,8, the amplitude of the “global signal” was measured as standard deviation of the mean signal from the whole brain region of interest (Figure 3). A significantly greater global signal standard deviation was observed when the SynCAM1 gene was knocked out (p=0.0003 SynCAM1, p=0.091 LRRTM1, p=0.24 interaction). A post-hoc T-test (two-sample, one-tailed, equal variance) indicated a significantly greater global signal in DKO versus SCKO.
DISCUSSION
Both the response to whisker stimulation and resting state functional connectivity were higher in mice with the gene encoding SynCAM1 knocked out. Unlike whisker stimulation, the functional connectivity pattern was non-significant and lost following global signal regression. However, the amplitude of the global signal itself was significant and showed both an effect of SynCAM1 knockout and (post-hoc) an increased effect in DKO (versus SCKO) was observed.
Human studies have shown in patients with schizophrenia an increased global resting state fMRI signal1. Unfortunately, the global changes observed there and in other studies remained difficult to understand in terms of mechanism2. Ideas for potential mechanisms may be derived from our work here. The primary effect we observed was both stimulation-response and globally at rest had increased activity in the SynCAM1 knockouts. SynCAM1’s protein is expressed during synaptogenesis across the brain4,5 and SynCAM1 reduces inhibitory drive in the hippocampus due to a reduction in excitatory inputs to interneurons. Its knockout may therefore alter the inhibitory balance and increase activity across the brain. LRRTM1’s protein modulates synaptic cell adhesion in glutamatergic neurons3, so its presence may prevent the worst effects of SynCAM1 loss. However, if both genes are lost, we observed increased global resting state activity suggesting a more severe change to function.
CONCLUSION
Mice with genes for SynCAM1 and LRRTM1 knocked out showed increased activation in stimulation and resting state fMRI. The strongest resting state fMRI result, of increased global signal, well matches the observed result in human schizophrenia patients. Our results suggest that global disruptions in functional connectivity may be related to disruptions of synaptic adhesion proteins in development.Acknowledgements
The authors would like to thank Elizabeth Lippard, Kristian Mortensen, Peter Herman, Daniel Coman, Bei Wang and Xiaoxian Ma for assistance with experiments. Supported by NIH grant R01 DA-018928 and a fellowship supporting GJT from NIH 2T32DA022975-06A1.References
1. Yang GJ, Murray JD, Repovs G, et al. Altered global brain signal in schizophrenia. Proc Natl Acad Sci U S A 2014;111(20):7438-7443.
2. Thompson GJ, Riedl V, Grimmer T, Drzezga A, Herman P, Hyder F. The whole-brain "global" signal from resting state fMRI as a potential biomarker of quantitative state changes in glucose metabolism. Brain Connect 2016;6(6):435-447.
3. Leach EL, Prefontaine G, Hurd PL, Crespi BJ. The imprinted gene LRRTM1 mediates schizotypy and handedness in a nonclinical population. J Hum Genet 2014;59(6):332-336.
4. Park KA, Ribic A, Laage Gaupp FM, et al. Excitatory Synaptic Drive and Feedforward Inhibition in the Hippocampal CA3 Circuit Are Regulated by SynCAM 1. J Neurosci 2016;36(28):7464-7475.
5. Robbins EM, Krupp AJ, Perez de Arce K, et al. SynCAM 1 adhesion dynamically regulates synapse number and impacts plasticity and learning. Neuron 2010;68(5):894-906.
6. Grandjean J, Schroeter A, Batata I, Rudin M. Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns. Neuroimage 2014;102 Pt 2:838-847.
7. Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 2009;44(3):893-905.
8. Wong CW, Olafsson V, Tal O, Liu TT. The amplitude of the resting-state fMRI global signal is related to EEG vigilance measures. Neuroimage 2013;83:983-990.
Figures