0246
MRI Connectivity Predictors of Post-Surgical Seizure Outcome in Temporal Lobe Epilepsy1Institute of Imaging Science, Vanderbilt University, Nashville, TN, United States, 2Neurosurgery, Vanderbilt University, Nashville, TN, United States, 3Biomedical Engineering, Vanderbilt University, Nashville, TN, United States, 4Electrical Engineering and Computer Science, Vanderbilt University, Nashville, TN, United States, 5Neurology, Vanderbilt University, Nashville, TN, United States
Synopsis
Surgical resection of the mesial temporal lobe is a common treatment of drug-resistant temporal lobe epilepsy (TLE). The ability to more accurately predict post-surgical seizure outcome in these patients is a significant clinical challenge. Towards this end, MRI functional and structural connectivity were used to identify a common pattern across a seizure propagation network in TLE patients with seizure free outcome. Then, in test patients with good and bad outcomes, similarity with the model pattern was significantly associated with seizure outcome. Therefore, this non-invasive method may predict seizure outcomes in TLE, which was not possible from the clinical assessments alone.
Purpose
Surgical resection of the mesial temporal lobe is a common treatment of drug-resistant temporal lobe epilepsy (TLE).1 While approximately 60-70% of these patients will become seizure free2, more accurate prediction of seizure outcome in these patients could prevent unsuccessful surgery, or identify alternative surgical targets or treatments. The goals of this work were 1) to use presurgical MRI to identify a common pattern of functional (FC) and structural (SC) connectivity in a set of patients with complete post-surgical seizure freedom as a model of favorable outcome, and 2) to validate this model by comparing the pattern to independent patients, including those with favorable and unfavorable outcomes.
Methods
We enrolled TLE patients who were scheduled to undergo mesial temporal resection (n = 25, 13F, 8 right TLE) and age and gender matched healthy controls (n = 35). Presurgical 3T MRI images were acquired using resting state functional MRI for FC (TR=2 s, 3x3x4 mm3, 600 vols) and diffusion weighted MRI for SC (b=1600 s/mm2, 92 directions, 2.5 x 2.5 x 2.5 mm3, 3 averages). One year post-surgical assessment showed 13 TLE patients with complete seizure freedom (Engel3 1A), four with good outcome as indicated by Engel 1B (auras only) or Engel 2 (rare disabling seizures, almost seizure free), and five with poor outcome as indicated by Engel 3 (worthwhile improvement) or Engel 4 (no worthwhile improvement). Three patients were less than 1 year post-surgery.
The seizure propagation network included three regions ipsilateral and contralateral to seizure onset (hippocampus, insula, thalamus), with two midline bilateral structures (precuneus and midcingulate). FC was calculated as the correlation between pairwise regional time series transformed to Z scores.4 For SC, FSL5 probabilistic fiber tracking was used to compute the fraction of total fibers from the seed to each of other regions normalized to the size of the two regions. Using the control population and their associated ages, patient FC and SC values were transformed to standard deviations from average of age matched control. A degree measure was then computed for each node of the network (8 FC and 8 SC) as the weighted sum of the connectivity to each of the other seven nodes in the network. Ipsilateral and within hemisphere connections were weighted more strongly than contralateral and between hemisphere connections.
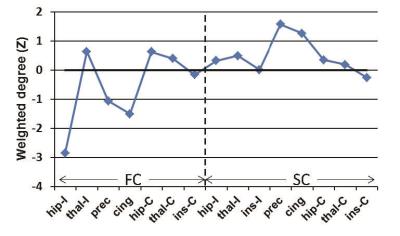
Principal components analysis was used to compute a common FC and SC weighted degree pattern using 8 of the 13 seizure free patients as the training set. Of the 16 potential nodes in the analysis (8 FC and 8 SC), the FC of the ipsilateral insula was not included in the principal component analysis or further comparisons due to high variability across the training set. Therefore, the result was a 15 point pattern of connectivity across this network associated with complete seizure freedom (seizure free model). Each patient's connectivity pattern was then compared to the seizure free model using two metrics: 1) Pearson correlation and 2) Euclidean distance.
Results
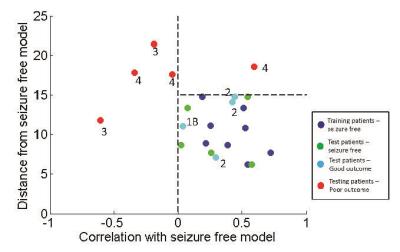
A common connectivity pattern was identified across a training set of 8 seizure free TLE patients as a model of favorable seizure outcome (Figure 1), and all patients were then compared to this model (Figure 2). The 8 seizure free training patients had positive correlation with and a Euclidean distance of less than 15 compared to the model (Figure 2, blue). Five of 5 seizure free patients (Engel 1A) in the test set fell within these limits as compared to the model (Figure 2, green). In addition, 4 of 4 patients with good seizure outcome (Engel 1B-2) also had properties similar to the model (Figure 2, cyan), while 5 of 5 patients with poor outcome (Engel 3-4) did not have properties similar to the model (Figure 2, red). Thus, in our test patients, similarity with the model pattern has a statistically significant association with seizure outcome (chi square with Yates correction = 14, p = 0.001).Discussion and Conclusions
In this work we identified a connectivity pattern across a seizure propagation network that shows promise as an indicator of seizure outcome after surgical treatment of TLE. We developed our method based on the assumption that the seizure free patients should share a pattern of common connectivity across this seizure propagation network, while deviation from this pattern will indicate potential for seizure recurrence. These preliminary results show that this method may distinguish poor outcome from good and seizure free outcome, which was not possible from the clinical assessments alone. Therefore, if further validation is successful, this non-invasive assessment may improve the use of surgical treatment of temporal lobe epilepsy.
Acknowledgements
This work was supported by NIH R01 NS75270 – VLM.References
1. Langfitt JT, Wiebe S. Early surgical treatment for epilepsy. Current opinion in neurology 2008;21:179-183.
2. Janszky J, Janszky I, Schulz R, et al. Temporal lobe epilepsy with hippocampal sclerosis: predictors for long-term surgical outcome. Brain 2005;128:395-404.
3. Engel J Jr, Van Ness PC, Rasmussen TB, Ojemann LM: Outcome with respect to epileptic seizures, in Engel J Jr (ed): Surgical Treatment of the Epilepsies, ed 2. New York: Raven Press, 1993, pp 609–621
4. Rogers BP, Morgan VL, Newton AT, Gore JC. Assessing functional connectivity in the human brain by fMRI. Magn Reson Imaging 2007;25:1347-1357.
5. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage 2012;62:782-790.
Figures