0238
Simultaneous GCaMP6 based fiber photometry and fMRI in rats1Institute of Neuroscience, Chinese Academy of Sciences, Shanghai, People's Republic of China, 2Department of Biomedical Engineering, Pennsylvania State University, PA, United States
Synopsis
Measuring neural activities simultaneously with fMRI using electrophysiology approaches has been valuable in elucidating neural basis of BOLD signals, but is also technically challenging due to interferences from MRI scanners. Optical recording of neural activities such as calcium signals has minimal interferences from MRI, and thus has opened new avenues of simultaneous acquisition of neural and BOLD signals. The current study first demonstrated the feasibility of using protein based calcium indicator (GCaMP6) to simultaneously and repeatedly acquire calcium and BOLD signals in the MRI scanner, with significant increases of both signals in response to visual stimulation.
Introduction
Functional MRI typically measure the blood oxygenation level dependent (BOLD) signal as a surrogate of neural activities. Since the BOLD signal is vascular in nature, a direct measurement of neural activity simultaneously with fMRI is highly desired to elucidate the neural mechanisms of the BOLD signal. To this date, most such studies utilized the simultaneous electrophysiology and fMRI approach (e.g., Logothetis et al.1 ), which suffers from significant interference from fMRI-related electromagnetic artifacts. A new artifact-free approach based on simultaneous optical and fMRI measurements was previously demonstrated using calcium sensitive dyes2. To take advantages of recent advances in genetically encoded calcium indicators, the current study first demonstrated the feasibility and reliability of simultaneous GCaMP6-based fiber photometry and fMRI in rats with a visual stimulation paradigm.Methods
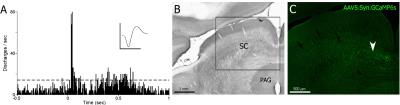
Seven Long-Evans rats underwent standard stereotaxic surgeries, and the visually responsive area of superior colliculus was identified using extracellular recording. AAV virus (AAV5.Syn.GCaMP6s, Penn Vector Core) was then injected into that area and optical fiber was implanted immediately above the injection site. After approximately 4 week recovery period, those rats were anesthetized using dexmedetomidine (initial bolus injection of 0.05 mg/kg and subsequent subcutaneous infusion 0.1 mg/kg/h), and imaged using a 7T Bruker scanner. During EPI scans (TR=1s, 15 slices, slice thickness 1mm, in plane resolution 0.5mm×0.5mm), visual stimulation (5Hz, 2s, 5s or 10s) was delivered through an blue LED coupled optical fiber placed right in front of animals’ left eyes. Calcium signals were acquired simultaneously during EPI scans through implanted optical fiber. Optical recording setup was adopted from Gunaydin et al.3 and calcium signals were synchronized with the MRI scanner. 4 out of 7 rats were imaged again several days after initial imaging sessions. fMRI data were analyzed with standard preprocessing and statistical analysis using SPM12. Calcium data were low pass filtered (<10 Hz) and detrended. To plot BOLD and calcium signals simultaneously, both signals were re-sampled to 10 Hz. BOLD time courses were averaged from time courses of significantly activated voxels of 3 slices (the slice of implanted optical fiber, one slice before and one slice after, most those voxels were adjacent to the tip of the implanted optical fiber) in each rat.Results
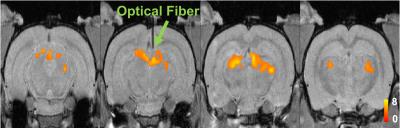
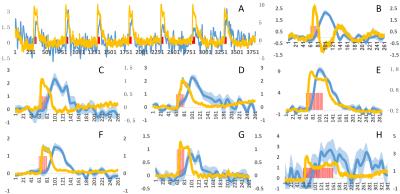
Visually responsive areas of superior colliculus were identified using extracellular recording (Fig. 1a) in each animal and GCaMP6s expression was verified histologically after animals were sacrificed (Fig. 1c). Visual stimulation lead to significant activations of BOLD signals in superior colliculus (Fig. 2). Importantly, simultaneously acquired calcium signals also showed robust activations that were temporally synchronized with BOLD signals of nearby activated voxels (Fig. 3). At single epoch level, the synchronized activation of BOLD and calcium signals was evident (Fig. 3a), which was also observed in averaged signals of each individual animal (Fig. 3b-h). The amplitudes of BOLD activation were similar across different animals (~2%), while the calcium signal amplitudes varied from 1% to 10%, possibly due to differences in AAV injection and placement of optical fibers.
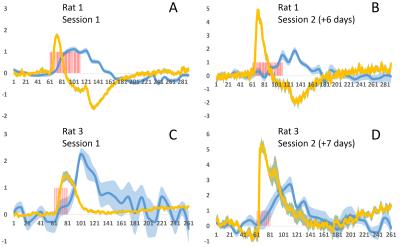
Unlike fluorescent dyes, GCaMP6 is virally expressed protein indicator which is very well suited for repeated and longitudinal measurements. This advantage was demonstrated in Fig. 4, where two imaging sessions separated by approximately one week in two animals both clearly showed BOLD and calcium activations with similar temporal patterns. Interestingly, calcium signal amplitudes of second sessions were higher compared to the first sessions, potentially due to increased GCaMP expression over time.
Discussion
To the best of our knowledge, the current study is the first to demonstrate the feasibility of GCaMP6 based simultaneous calcium fiber photometry and fMRI. This approach allows simultaneous recording of neural activity along with fMRI without the interference from MRI. Compared to previous dye based calcium fiber photometry and fMRI studies, the current study utilized GCaMP6, a newly improved genetically encoded calcium indicator. The benefit of using GCaMP6 instead of dyes is that it allows repeated and longitudinal recording, as demonstrated in the current study (Fig. 4). Other possibilities afforded by GCaMP6 include neuron-type specific recording, which may greatly advance our current understanding of neurovascular coupling. Overall, the current study established the methodology of GCaMP based simultaneous calcium fiber photometry and fMRI in rats, and such method opens new avenues to examine the neural basis of BOLD fMRI measurement.Acknowledgements
The work was supported by the National Institutes of Health, Grant Numbers R01MH098003 (PI: Nanyin Zhang, PhD) from the National Institute of Mental Health and R01NS085200 (PI: Nanyin Zhang, PhD) from the National Institute of Neurological Disorders and Stroke.References
1.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001 Jul 12;412(6843):150-7.
2.Schulz K, Sydekum E, Krueppel R, Engelbrecht CJ, Schlegel F, Schröter A, Rudin M, Helmchen F. Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nat Methods. 2012 Jun;9(6):597-602.
3.Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC, Deisseroth K. Natural neural projection dynamics underlying social behavior. Cell. 2014 Jun 19;157(7):1535-51.
Figures