0224
Short 15-min surveillance-protocol multiphasic contrast enhanced MRI for hepatocellular carcinoma detection in cirrhosis1Radiology, UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
Patient with cirrhosis are at increased risk of developing hepatocellular carcinoma (HCC) and routine surveillance imaging is recommended every 6 months. While MRI has high sensitivity for HCC detection, its routine use is controversial due to its long exam time, high cost, and limited access. In this prospective study, we demonstrated that a short 15-min surveillance MRI has similar HCC detection performance as the standard 45-min diagnostic MRI in patients with cirrhosis. Therefore, a short surveillance MRI may allow for a more efficient and cost-effective alternative to the current standard-of-care MRI for HCC surveillance.
Clinical Question
Can a short 15-min surveillance MRI protocol be as effective as standard-of-care 45-min diagnostic MRI protocol in hepatocellular carcinoma (HCC) detection in an at-risk cirrhotic patient population?Impact
HCC is currently the 8th leading cause of cancer-related death in the U.S. [1] and is the fastest growing cancer in mortality [2]. The major risk factor is cirrhosis, for which routine imaging surveillance is recommended. At our institution, ~3,000 cirrhotic patients are undergoing surveillance ultrasound every 6 months. In recent meta-analyses [3-5], ultrasound (US) and MRI had a per-patient HCC detection sensitivity of 60-63% and 83-92%, respectively. Despite higher sensitivity, MRI for routine HCC surveillance is controversial due to its long examination time, high cost, and limited scanner access. Implementation of a short MRI protocol focusing on HCC detection may allow for a more cost-effective surveillance compared to the standard-of-care MRI or US.Approach
We propose a short HCC surveillance MRI protocol comprised of a localizing coronal T2-weighted and multiphasic contrast enhanced (MCE) axial T1-weighted fat-suppressed sequences only. To justify its clinical implementation, we conducted a non-inferiority study to evaluate the HCC detection performance of the surveillance MRI compared to our standard-of-care diagnostic MRI.
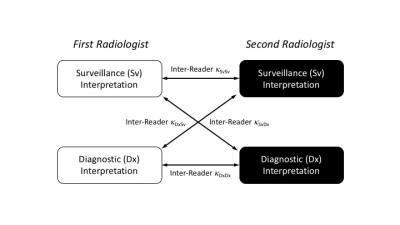
In this prospective observational study, consecutive adult patients with cirrhosis (by clinical history or imaging) undergoing liver MRI were enrolled. Patients with previously treated HCC were excluded. Our standard diagnostic MRI protocol was performed in all patients: non-contrast coronal T2-, axial T2- (with/without fat saturation), axial T1- (in-/opposed-phase), and diffusion-weighted sequences, followed by axial T1-weighted fat suppressed MCE (i.e. pre-contrast, arterial, portal, venous, and equilibrium) sequences using extracellular gadolinium contrast. Using the Liver Imaging and Reporting Data System (LI-RADS) algorithm [6], two radiologists independently interpreted two sets of images of each patient: the surveillance-set ("Sv") consisting of the coronal T2-weighted and MCE images only, and the diagnostic-set ("Dx") consisting of all non-contrast and MCE images. For each liver lesion, its likelihood of HCC was scored using a 5-point ordinal scale: LR-1 to LR-5, where LR-1 is definitely benign and LR-5 definitely HCC. The inter-reader agreement between the Sv and Dx LI-RADS interpretations was assessed as illustrated in Figure 1 using multi-reader Fleiss kappa (κSvDx & κSvDx), and was compared to the inter-reader agreement between the two Dx interpretations (κDxDx) as the reference benchmark. A non-inferiority test was performed for their differences, κDxDx – κSvDx and κDxDx – κDxSv, with non-inferiority margin of 0.2 and significance level α=0.05. The LI-RADS score agreement was assessed per-lesion as well as per-patient basis (i.e. highest LI-RADS score of all liver lesions within a given patient).
Gains and Losses
The proposed HCC surveillance MRI can be completed within 15 min as opposed to 45-min for the current standard-of-care diagnostic MRI at our institution, thereby shortening the examination time by 66%. This increased efficiency in turn may result in the following gains: reduction in the examination cost, greater scanner access, and a higher HCC detection rate compared to ultrasound surveillance. As shown below, there is no significant loss in diagnostic performance compared to standard-of-care MRI, as the radiological interpretation of surveillance MRI does not significantly differ from that of the standard MRI.Preliminary Data
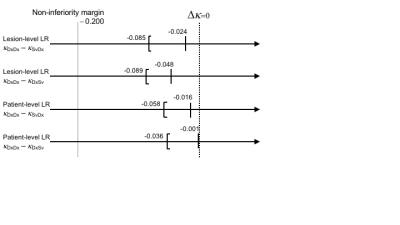
In this prospective study, 100 MRI exams in 100 unique HCC treatment-naïve cirrhotic patients were performed during a 15-week period in 2016. Six exams were excluded: 4 for severe motion artifacts, 1 for use of hepatobiliary contrast agent, and 1 for a subsequently-confirmed previous HCC treatment. A total of 96 lesions in 94 patients were included in the data analysis. The inter-reader agreement between two independent radiologists is summarized in Table 1. The agreement between the two Sv interpretations, as well as between the two Dx interpretations, were moderate and similar with κSvSv, κDxDx = 0.550-0.645, representing the inherent reader variability in LI-RADS interpretation. The inter-reader agreement between the Sv and Dx sets (κSvDx & κDxSv) were also similar, with κSvDx, κDxSv = 0.550-0.586. The agreement between the Sv and Dx sets (κSvDx, κDxSv) was within the non-inferiority margin of Δκ = 0.2 from the agreement of the Dx sets (κDxDx, the reference benchmark), in lesion- and patient-level absolute LI-RADS score, as illustrated in Figure 2.Discussion/Conclusion
The radiological interpretation of a short HCC surveillance MRI protocol is similar to that of the standard diagnostic MRI protocol in patients with cirrhosis at risk for HCC. Therefore, a short surveillance MRI may allow for a more efficient and cost-effective alternative to the current standard-of-care MRI. However future randomized control trials are necessary to directly evaluate diagnostic sensitivity/specificity as well as cost-effectiveness of surveillance MRI.Acknowledgements
We would like to thank UT Southwestern Body MRI and Abdominal Imaging Division attending radiologists as well as trainees for participating in this study.References
1. U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2013 Incidence and Mortality Web-based Report., in Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Available at: http://www.cdc.gov/uscs. . 2016.
2. Ryerson, A.B., et al., Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer, 2016. 122(9): p. 1312-37.
3. Colli, A., et al., Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol, 2006. 101(3): p. 513-23.
4. Singal, A., et al., Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther, 2009. 30(1): p. 37-47.
5. Lee, Y.J., et al., Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology, 2015. 275(1): p. 97-109. 6. American College of Radiology. Quality and safety resources: Liver Imaging–Reporting and Data System. 2014 [cited 2016 10/1]; Available from: http://www.acr.org/Quality-Safety/Resources/LIRADS.
Figures
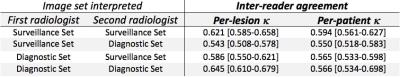
Table 1:
Inter-reader agreement between two radiologists providing LI-RADS interpretations for both the surveillance- and diagnostic-image sets. Legends: [ , - ] represents two-sided 95% confidence interval.