0221
Improving white matter lesion conspicuity in multiple sclerosis using patient-specific optimization of 3D FLAIR1Diagnostic and Interventional Imaging, University of Texas Health Science Center at Houston, Houston, TX, United States, 2Philips Healthcare, Cleveland, OH, United States, 3Neurology, University of Texas Health Science Center at Houston, Houston, TX, United States
Synopsis
Fluid-attenuated inversion recovery (FLAIR) imaging is widely used in multiple sclerosis (MS) scan protocols for its good lesion to tissue contrast. Optimization of 3D FLAIR acquisition parameters for the individual patient could further improve lesion conspicuity. In this work, tissue contrast between lesions and white matter for 3D FLAIR was optimized in the same scan session based on fast measurement of the relaxation times and proton density. Results on 16 MS patients show ~30% improved lesion contrast with the patient-specific acquisition parameters compared to the fixed-parameter 3D FLAIR sequence.
INTRODUCTION
MRI is the most sensitive imaging tool for diagnosing and monitoring patients with multiple sclerosis (MS). MS white matter (WM) lesions (WMLs) are detected as hyperintensity on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images. The improved spatial resolution in 3D FLAIR 1 may further enhance the detection of small and subtle lesions.2 However, contrast optimization of modern single-slab 3D FLAIR sequences remains a challenge.3,4 Moreover, heterogeneity in WM injury between patients renders tuned sequences suboptimal for individual studies. In this work we present a method for patient-specific and real-time optimization of the scan parameters of 3D FLAIR to improve visualization of WMLs in MS.METHODS
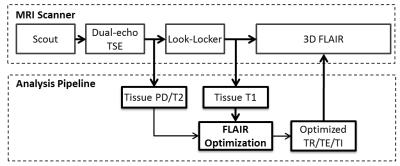
This study included 16 MS patients (age 46±11 years; 13 females; 14 relapsing-remitting and two secondary progressive MS). After IRB approval and obtaining written consent from the participants, MRI scans were performed on a 3.0 T Philips Ingenia system. The study targeted the optimization of the contrast of WMLs by calculating optimal values for the inversion time (TI) and echo time (TE) of 3D FLAIR. Optimization was based on patient-specific measurements of the T1 and T2 relaxation times and the relative proton density (PD) of the individual’s brain tissue performed during the imaging session. Noting that WMLs typically show longer relaxation times similar to those of gray matter (GM), maximizing the GM-WM contrast can help increase the WML-WM contrast. Hence, the criterion for optimizing the scan parameters was to minimize the expression: SCSF/(SGM-SWM). Here S denotes the MRI signal at the effective echo time.
The imaging protocol included T1 mapping performed using a Look-Locker sequence 5 (TR/TE/FA = 6.4ms/3.1ms/7°, 67 samples, scan time 51 s). To minimize the additional scan time requirements, PD and T2 mapping utilized a multi-slice dual-echo turbo-spin echo protocol (TR/TE1/TE2 = 6800/9.5/90 ms), which is part of the standard clinical MS protocol at our site. Brain segmentation and relaxation time calculations were performed immediately after data acquisition (Fig 1), and the median values of T1, T2, and PD (relative to CSF) of WM and GM were computed and used for parameter optimization. The optimized scan parameters were imported back to the MRI scanner and applied for FLAIR acquisition. A standard FLAIR protocol (TR/TE/TI = 4800/304/1650 ms, contrast-equivalent TE=130 ms) was also acquired for comparison, with otherwise identical scan parameters: FOV=256×256×180 mm3, voxel size = 1×1×1 mm3, SENSE acceleration factor=2.5x2.5, echo train length=167, echo spacing=3.3 ms, and total scan time 5:31 min. Optimization of TR was restricted to keep comparable scan time in the two FLAIR scans.
Region-of-interest analysis was performed in up to 10 randomly chosen WMLs and neighboring WM tissue, and in the lateral ventricles. The contrast ratios between WMLs and both WM and CSF were calculated to assess the performance of the fixed-parameter and the patient specific FLAIR protocols.
RESULTS
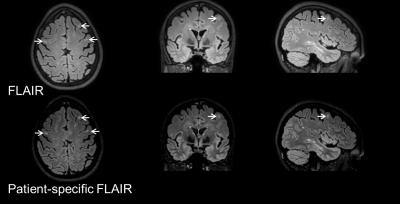
Automated optimization of FLAIR was successfully performed in all scans. The optimized scan parameters were computed in 137 ± 66 s. The average values for the scan parameters in the patient-specific protocol were: TI = 1577±34 ms, and contrast equivalent TE of 158±30 ms. Fig. 2 demonstrates the improved lesion conspicuity obtained with patient-specific 3D FLAIR compared to standard 3D FLAIR. Using patient-specific optimization, the WML-WM contrast ratio increased by approximately 30% compared to the fixed FLAIR protocol (0.74±0.25 vs. 0.56±0.12; P<0.01). The CSF signal was higher in the patient-specific protocol, reducing the WML-CSF contrast ratio from 14±4.6 to 5.7±3.7 (P<10-4). However, CSF was still suppressed compared to surrounding tissue, and periventricular lesions were easily identified.CONCLUSION
The proposed patient-specific optimization of the 3D FLAIR scan parameters improves the contrast between WMLs and brain normal-appearing WM. This increase in lesion/brain contrast with the high dynamic range and spatial resolution of 3D FLAIR could enhance the detection and classification of WM lesions in MS. Importantly; this comes with very moderate increase in the scan time (<1 min for the additional Look-Locker scan, and ~2 min for post-processing and scan parameter optimization). Use of look-up table for parameter optimization will significantly reduce the computational time cost. Future large-scale studies will investigate the potential of this patient-specific approach for enhanced detection of lesions in the brain and the spinal cord in MS.Acknowledgements
This work was supported by the Clinical Translational Science Award (CTSA) Grant UL1 TR000371 from the NIH National Center for Advancing Translational Sciences, and the Chair in Biomedical Engineering Endowment Funds. We thank Vipulkumar Patel and Corina Donohue for assistance in conducting the MRI experiments.References
1. Kallmes DF, Hui FK, Mugler JP. Suppression of cerebrospinal fluid and blood flow artifacts in FLAIR MR imaging with a single-slab three-dimensional pulse sequence: initial experience 1. Radiology. 2001;221:251–255.
2. Bink A, Schmitt M, Gaa J, Mugler JP, Lanfermann H, Zanella FE. Detection of lesions in multiple sclerosis by 2D FLAIR and single-slab 3D FLAIR sequences at 3.0 T: initial results. Eur Radiol. 2006;16:1104–1110.
3. Polak P, Magnano C, Zivadinov R, Poloni G. 3D FLAIRED: 3D fluid attenuated inversion recovery for enhanced detection of lesions in multiple sclerosis. Magn Reson Med. 2012;68:874–81.
4. Madhuranthakam AJ, Sarkar SN, Busse RF, Bakshi R, Alsop DC. Optimized double inversion recovery for reduction of T 1 weighting in fluid-attenuated inversion recovery. Magn Reson Med. 2012;67:81–88.
5. Henderson E, McKinnon G, Lee TY, Rutt BK. A fast 3D Look-Locker method for volumetric T1 mapping. Magn Reson Imaging. 1999;17:1163–1171.
Figures