0218
Multiple sclerosis lesions are softer than surrounding white matter: An MR elastography study1Department of Biomedical Engineering, University of Delaware, Newark, DE, United States, 2Kessler Foundation, East Hanover, NJ, United States, 3Department of Kinesiology and Community Health, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 4Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 5Interdisciplinary School of Health Sciences, University of Ottawa, Ottawa, ON, Canada, 6Department of Physical Therapy, University of Alabama at Birgmingham, Birmingham, AL, United States
Synopsis
Mechanical properties of the brain measured with magnetic resonance elastography (MRE) have proven sensitive to tissue health in neurological conditions, including multiple sclerosis (MS). In this study, we use high-resolution MRE to examine the mechanical properties of focal lesions in subjects with MS to determine if they exhibit viscoelastic signatures that differ from surrounding white matter. In a sample of fourteen subjects, we found that lesions are significantly softer than surrounding white matter. This finding suggests MRE is sensitive to tissue disruption localized to focal lesions, and may provide novel measures of tissue health in the assessment of MS.
Introduction
Mechanical properties of brain tissue measured with magnetic resonance elastography (MRE1) have proven sensitive to a number of neurological conditions2. Notably, MRE has detected neural tissue softening in disease courses of multiple sclerosis (MS): from clinically-isolated syndrome3, to relapsing-remitting MS4, and to progressive MS5. However, these studies have focused on global effects of the disease on tissue integrity; the focal lesions associated with MS have not yet been characterized by MRE. MS lesions exhibit signatures of inflammatory demyelination6, and the ability to characterize lesions in vivo could improve treatment of the disease. In this study, we use high-resolution MRE methodology7,8 to characterize the viscoelasticity of focal lesions in subjects with MS and compare with surrounding white matter.Methods
Participants: We included baseline data from subjects that were participating in two separate ongoing studies that had all necessary image series for the analysis in this work. Our total sample included 14 participants with a confirmed diagnosis of MS (13/1 female/male; age range = 25-63 years; median age = 48 years).
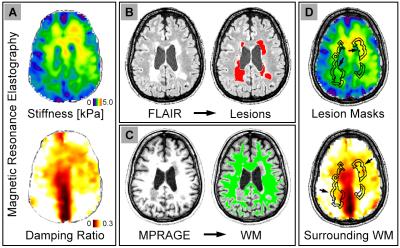
Imaging: Each participant completed an imaging session on a Siemens 3T Trio scanner with 12-channel head coil (Siemens Medical Solutions; Erlangen, Germany). The data acquisition protocol included high-resolution MRE, T1-weighted MPRAGE (0.9 mm isotropic resolution: 230x230x172 total-FOV (sagittal); 256x256x192 matrix; TR/TE/TI = 1900/2.32/900 ms) and T2-weighted FLAIR (1.0 mm isotropic resolution: 250x250x160 total-FOV (sagittal); 250x250x160 matrix; TR/TE/TI = 6000/388/2200 ms).
The MRE scan used a 3D multislab, multishot spiral sequence with 1.6 mm isotropic resolution7: 240x240x96 mm total-FOV; 150x150x60 matrix (oblique axial); TR/TE = 1800/73 ms. Vibrations were applied at 50 Hz to the head using a pneumatic actuator with soft pillow-driver (Resoundant, Inc.; Rochester, MN). Viscoelastic property maps were generated from MRE displacement images using nonlinear inversion (NLI8), which returned viscoelastic shear stiffness and damping ratio.
Analysis: The analysis procedure is outline in Figure 1. (1) We segmented lesions from FLAIR images using an automated lesion estimation package9. (2) We then registered T1-weighted images to the FLAIR images and segmented white matter using FAST in FSL10. (3) To create masks of surrounding tissue, lesion masks were dilated in Matlab, and all added voxels with at least 70% white matter fraction were included. Dilation was chosen to approximate the same volume in the white matter masks as in the lesion masks. (4) MRE property maps were also registered to FLAIR images and average properties of lesions and surrounding white matter were calculated.
Results and Discussion
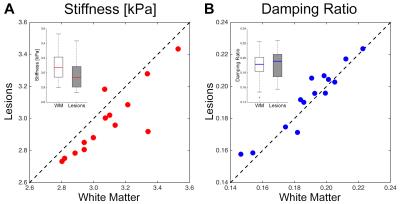
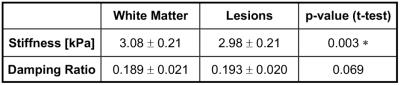
Plots comparing the stiffness and damping ratio of lesions and surrounding white matter in each subject are presented in Figures 2 and 3, respectively. Average properties are presented in Table 1 along with p-value from comparison with paired t-tests.
We found that lesions were significantly softer than surrounding white matter ($$$\Delta$$$=-0.11 kPa; -3.4%; p = 0.003*). This finding agrees with the reports of global softening of the brain in MS4,5, and with MRE studies of animal MS models that observed softening in demyelination11 and inflammation12, which are present in lesions. We found higher damping ratio in lesions, though the relationship was not statistically significant in our sample ($$$\Delta$$$=0.003; 1.9%; p = 0.069). Previous studies found phase angle (analogous to damping ratio) to be lower in demyelination11 and unchanged in inflammation12; thus, our data suggest different relationships between microstructure and viscoelasticity in lesions compared with diffuse reorganization affecting global white matter.
Conclusions
This is the first study that has examined the local mechanical properties of focal lesions in MS. The ability to localize mechanical measures to focal lesions points to the promise of MRE in the characterization and treatment of MS in its earliest stages before significant global degeneration. Future work will seek to evaluate the role of lesional properties in clinical outcome measures, and how these MRE measures can be used to improve treatment of MS.Acknowledgements
This work was partially supported by grants from the National Multiple Sclerosis Society: IL-1503-03395 and PR-1411-02096, and the Consortium of Multiple Sclerosis Centers. Partial support for MRE data collection was provided by NIH/NIBIB grants R01-EB018230 and R01-EB001981.References
[1] Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic Resonance Elastography by Direct Visualization of Propagating Acoustic Strain Waves. Science 1995;269:1854–1857.
[2] Sack I, Jöhrens K, Wuerfel J, Braun J. Structure-Sensitive Elastography: on the Viscoelastic Powerlaw Behavior of in Vivo Human Tissue in Health and Disease. Soft Matter 2013;9:5672–5680.
[3] Fehlner A, Behrens JR, Streitberger K-J, Papazoglou S, Braun J, Bellmann-Strobl J, Ruprecht K, Paul F, Wuerfel J, Sack I. Higher-Resolution MR Elastography Reveals Early Mechanical Signatures of Neuroinflammation in Patients with Clinically Isolated Syndrome. J Magn Reson Imaging 2016;44:51–58.
[4] Wuerfel J, Paul F, Beierbach B, Hamhaber U, Klatt D, Papazoglou S, Zipp F, Martus P, Braun J, Sack I. MR-Elastography Reveals Degradation of Tissue Integrity in Multiple Sclerosis. NeuroImage 2010;49:2520–2525.
[5] Streitberger K-J, Sack I, Krefting D, Pfüller C, Braun J, Paul F, Wuerfel J. Brain Viscoelasticity Alteration in Chronic-Progressive Multiple Sclerosis. PLoS One 2012;7:e29888.
[6] Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of Multiple Sclerosis Lesions: Implications for the Pathogenesis of Demyelination. Ann Neurol 2000;47:707–717.
[7] Johnson CL, Holtrop JL, McGarry MDJ, Weaver JB, Paulsen KD, Georgiadis JG, Sutton BP. 3D Multislab, Multishot Acquisition for Fast, Whole-Brain MR Elastography with High Signal-to-Noise Efficiency. Magn Reson Med 2014;71:477–485.
[8] McGarry MDJ, Van Houten EEW, Johnson CL, Georgiadis JG, Sutton BP, Weaver JB, Paulsen KD. Multiresolution MR Elastography Using Nonlinear Inversion. Med Phys 2012;39:6388–6396.
[9] Wetter NC, Hubbard EA, Motl RW, Sutton BP. Fully Automated Open-Source Lesion Mapping of T2-FLAIR Images with FSL Correlates with Clinical Disability in MS. Brain Behav 2016;6:e00440.
[10] Zhang Y, Brady M, Smith SM. Segmentation of Brain MR Images Through a Hidden Markov Random Field Model and the Expectation-Maximization Algorithm. IEEE T Med Imaging 2001;20:45–57.
[11] Schregel K, Wuerfel E, Garteiser P, Gemeinhardt I, Prozorovski T, Aktas O, Merz H, Petersen D, Wuerfel J, Sinkus R. Demyelination Reduces Brain Parenchymal Stiffness Quantified in Vivo by Magnetic Resonance Elastography. P Natl Acad Sci USA 2012;109:6650–6655.
[12] Riek K, Millward JM, Hamann I, Mueller S, Pfueller CF, Paul F, Braun J, Infante-Duarte C, Sack I. Magnetic Resonance Elastography Reveals Altered Brain Viscoelasticity in Experimental Autoimmune Encephalomyelitis. NeuroImage Clin 2012;1:81–90.
Figures