0217
Corticosteroid Treatment Fails to Prevent Long-term Axonal Loss Assessed by Diffusion Basis Spectrum Imaging1Radiology, Washington University School of Medicine, St. Louis, MO, United States, 2Radiology, The First Affiliated Hospital of Nanchang University, Jiangxi, People's Republic of China, 3Biomedical Engineering, Washington University in St. Louis, St. Louis, MO, United States, 4Biostatistics, Washington University School of Medicine, St. Louis, United States, 5Neurology, Washington University School of Medicine, St. Louis, MO, United States, 6The Hope Center for Neurological Disorders, Washington University School of Medicine, St. Louis, MO, United States
Synopsis
Glucocorticoids are commonly used to treat acute optic neuritis. Herein, we employed longitudinal diffusion basis spectrum imaging (DBSI) to examine and compare optic nerve integrity in EAE with PBS or Dexamethasone treatment. Our results indicate that anti-inflammatory treatment with corticosteroids alone is not sufficient to prevent eventual axonal loss in mice, and may have relevance for treatment of MS exacerbations with corticosteroids. DBSI could serve as an outcome measure to monitor longitudinal disease progression and to help stratify treatments.
Introduction
Corticosteroids have been widely used as an anti-inflammatory agent to treat acute optic neuritis (ON) in humans. The optic neuritis treatment trial (ONTT) concluded that, although they hasten recovery, corticosteroids do not improve long-term visual function recovery.1 Axonal loss is likely the primary substrate of the irreversible visual impairment that can occur post-ON. Longitudinal quantification of axonal integrity of the optic nerves from mice with ON treated with corticosteroids could shed light on the evolving pathophysiology and disease progression of ON treated in this manner. Previously, we have introduced diffusion basis spectrum imaging (DBSI) to assess coexisting inflammation, demyelination, and axonal injury and loss in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) mice,2-6 suggesting DBSI could serve as an outcome measure with disease progression and treatment efficacy. In the current study, we applied DBSI to assess whether dexamethasone (Dex) protects axons from irreversible damage in the EAE model.Materials and Methods
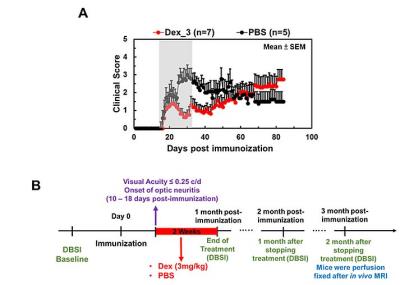
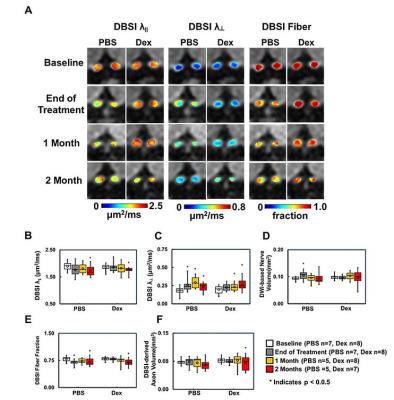
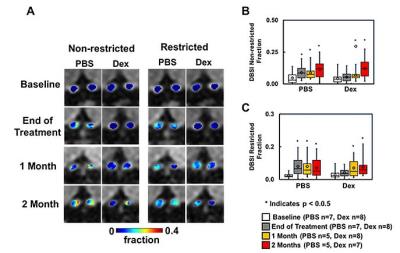
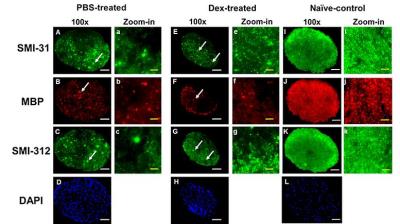
Animal model: EAE was induced with MOG35-55 peptide in incomplete Freund's adjuvant emulsion in 15 C57BL/6 female mice. After immunization, daily clinical score (CS) and body weight were assessed. Visual Acuity (VA) & treatment strategy: Daily VA was assessed after immunization. When VA ≤ 0.25 cycle/degree (the definition of onset ON), EAE mice were injected with Dex (3mg/kg, n=8) or PBS (n=7) intraperitoneally for two weeks (Fig. 1B). DBSI: A pair of active-decoupled volume and surface coils were used. A 25-direction diffusion-weighted imaging (DWI) was performed at baseline (naïve, before immunization), end of treatment, and one and two months after treatment (Fig. 1B) on a 4.7-T Agilent small-animal MR scanner utilizing a multiple-echo spin-echo diffusion-weighted sequence.7 All images were obtained with the following acquisition parameters: TR = 1.5 s, TE = 35 ms, inter-echo delay = 20.7 ms, Δ = 18 ms, δ = 6 ms, maximal b-value = 2,200 s/mm2, with the same FOV = 22.5 × 22.5 mm2, slice thickness = 0.8 mm, and matrix size = 192 × 192 (before zero-filling). The number of PBS- or Dex-treated EAE mice were not consistent because some EAE mice died before the last DBSI scan (Fig. 2 and 3). Data analysis: A lab-developed DBSI code was performed on DWI data to estimate DBSI derived λǁ, λ⊥, restricted (putative cellularity) and non-restricted isotropic (putative edema or extracellular space) diffusion tensor fractions. Histology: mice were perfusion fixed immediately after the last in vivo MR measurements for immunohistochemistry (IHC) staining of SMI-312, MBP, SMI-31, and DAPI.Results
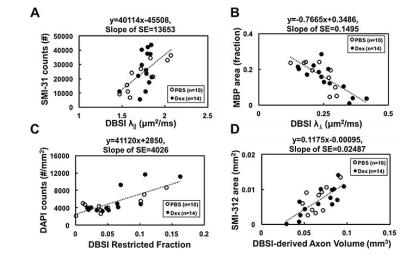
The working dosage of 3mg/kg Dex was determined by lower CS (reduced severity) than PBS-treated EAE during treatment (Fig. 1A), whereas the CS time course with ten-fold lower dosage, 0.3 mg/kg, was comparable to PBS-treated EAE mice (data not shown). Reduced DBSI λ∥ and increased DBSI λ⊥, and increased non-restricted, and restricted isotropic diffusion fractions were shown in PBS-treated ON but not in Dex-treated ON at the end of treatment (Fig. 2 and 3), suggesting axonal injury, demyelination, and edema and inflammatory cell infiltration in PBS-treated ON but not in Dex-treated ON. After treatment ended, DBSI metrics of axonal injury, demyelination, and inflammatory vasogenic edema and cell infiltration were seen in both PBS- and Dex-treated ON (Fig. 2 and 3). Reduced DBSI-derived axonal volume (tissue volume × fiber fraction) at the last time point (two months after cessation of treatment) in both PBS- and Dex-treated groups suggested axonal loss, i.e., lacking long-term axonal protection of short-term Dex treatment (Fig. 2 D – F). The IHC results revealed axonal injury (reduced SMI-31 intensity, A, a, E, e), demyelination (reduced MBP intensity, B, b, F, f), axonal loss (reduced SMI-312 intensity, C, c, G, g), axonal swelling (Fig. 4, white arrows) and cell infiltration (increased DAPI intensity, D) in both PBS- and Dex-treated ON (Fig. 4). The regression analysis of DBSI metrics and corresponding IHC markers suggested that DBSI metrics correctly reflected coexisting pathologies of ON. The open (PBS) and filled (Dex) symbols overlapped suggesting comparable severity between PBS-treated and Dex-treated optic nerves (Fig. 5).Conclusion
Our results are in accord with the previous ONTT findings that a short course of corticosteroid treatment of human ON does not protect optic nerves long-term. DBSI results indicate that anti-inflammatory treatment with corticosteroids alone is not sufficient to prevent eventual axonal loss in mice, and may have relevance for treatment of MS exacerbations with corticosteroids. DBSI could serve as an outcome measure to monitor longitudinal disease progression and to help stratify treatments.Acknowledgements
Supported in part by NIH R01-NS047592, P01-NS059560, and NMSS RG 5258-A-5, RG 1507-05315References
1. Gal RL, Vedula SS, Beck R. Corticosteroids for treating optic neuritis. The Cochrane database of systematic reviews 2012;CD001430.
2. Chiang CW, Wang Y, Sun P, et al. Quantifying white matter tract diffusion parameters in the presence of increased extra-fiber cellularity and vasogenic edema. NeuroImage 2014;101:310-319.
3. Lin T, Hallman M, Cusick MF, et al. Axon Loss as an Outcome Measure for Assessing Therapeutic Efficacy. Proc Intl Soc Mag Reson Med 24 (2016) 2016;0245.
4. Wang X, Cusick MF, Wang Y, et al. Diffusion basis spectrum imaging detects and distinguishes coexisting subclinical inflammation, demyelination and axonal injury in experimental autoimmune encephalomyelitis mice. NMR in biomedicine 2014;27:843-852.
5. Wang Y, Wang Q, Haldar JP, et al. Quantification of increased cellularity during inflammatory demyelination. Brain : a journal of neurology 2011;134:3590-3601.
6. Wang Y, Sun P, Wang Q, et al. Differentiation and quantification of inflammation, demyelination and axon injury or loss in multiple sclerosis. Brain : a journal of neurology 2015;138:1223-1238.
7. Tu TW, Budde MD, Xie M, et al. Phase-aligned multiple spin-echo averaging: a simple way to improve signal-to-noise ratio of in vivo mouse spinal cord diffusion tensor image. Magnetic resonance imaging 2014;32:1335-1343.
Figures