0206
Full Brain Vasculature Analysis using Gradient/Spin-Echo Multi-band EPIK: Tumour Evaluation with MR-PET1Institute of Neuroscience and Medicine - 4, Forschungszentrum Jülich, Jülich, Germany, 2Department of Neurology, Faculty of Medicine, RWTH Aachen University, JARA, Aachen, Germany, 3Department of Electrical and Computer Systems Engineering, and Monash Biomedical Imaging, School of Psychological Sciences, Monash University, Melbourne, Australia, 4Institute of Biomedical Engineering and Biophysics, Science Faculty of University of Lisbon, Lisbon, Portugal
Synopsis
Perfusion weighted imaging (PWI) using dynamic susceptibility contrast (DSC) imaging is a widely used technique in tumour imaging. The use of multi-echo DSC, gradient and spin echo (GESE), allows one to obtain vasculature information. However, trade-off between number of echoes, spatial resolution and brain coverage is required. In this work, the use of EPI with keyhole (EPIK) combined with multi-band is proposed to obtain a whole brain multi-echo GESE-DSC in clinically relevant acquisition times. The method was applied in a cohort of brain tumour patients in a MR-PET scanner enabling localisation of the tumour based on metabolic information from PET.
Introduction
Multi-echo DSC is an
attractive method for the assessment of brain vascular information via gradient
and spin echo (GESE) sequences1. However, it results in a reduced number of
slices when large matrix sizes with multi-echoes are acquired. The use of
simultaneous multi-slice (SMS) excitation together with the blipped-CAIPI
method has been proposed to overcome such limitation in the context of DSC2,3. However, in Ref. 3 only one GE and one SE contrast were acquired, which
limited the T1 leakage correction methods4. Thus, the goal of
this work is to develop a multi-echo DSC sequence using multi-band EPIK which
enables acquisition of two GEs and one SE whilst maintaining whole brain
coverageMaterial and Methods
Data from five tumour patients were included in this study. MR acquisitions were performed on a 3T MR-BrainPET scanner (MAGNETOM Trio, Siemens Medical Solutions, Erlangen, Germany). DSC data were acquired without pre-bolus and using the EPIK scheme5-7 combined with the multi-band technique8. DSC protocol parameters were as follows: three echoes (TE1/TE2/TE3=SE =13/36/117 ms); TR = 1500 ms; iPAT = 2; SMS= 2; partial Fourier = 5/8; matrix-size = 128×128×20; pixel size 1.9×1.9×5 mm3. Diffusion-weighted imaging (DWI) was performed using three b-values (0, 500 and 1000 s/mm2) and one gradient direction, TE/TR = 96/4900 ms, matrix size 192×192×25 and pixel size 1.2×1.2×5 mm3. Pre/post contrast MPRAGE images were acquired. Simultaneously with the MR measurements, an [18F]-FET PET scan was carried out. PET data9 were reconstructed using OP-OSEM3D software with 4 subsets and 32 iterations and the images were normalized and corrected for attenuation, scatter, dead time and random, resulting in images with voxels of 1.25×1.25×1.25 mm3 and matrix size of 256×256×153. After data acquisition, GE and SE DSC data were converted to contrast tissue concentration (DR2GE, DR2SE) and corrected for T1 effects using the three-echo approach suggested in Ref. 10. DWI data were processed in order to calculate the apparent diffusion coefficient (ADC). Vessel size images (VSI) were generated with the following formula11:
VSI = 0.867 (CBV ADC)1/2 ΔR2GE/(ΔR2SE3/2),
where CBV is the cerebral blood volume which was normalized to 3% in the normal apparent white matter. In order to perform a quantitative comparison, volumes-of-interest (VOIs) were placed in the grey matter, thalamus, white matter and tumour. Tumour volumes were defined in the PET summed image from 20 to 40 min p.i. by a 3D auto-contouring process using a tumour-to-brain ratio12 (TBR) of ≥ 1.6. All the processing was performed using developed in-house Matlab script.
Results and Discussion
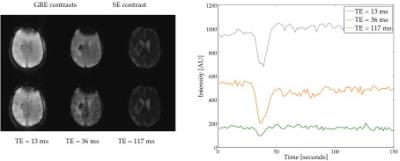
The proposed sequence allows
one to track the contrast agent bolus passage in all echoes (Fig. 1). In this
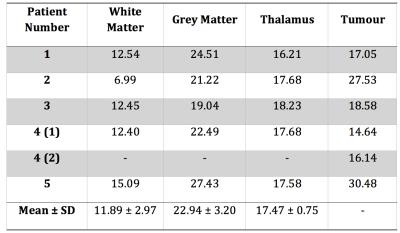
way, user slice positioning dependency is avoided. Analysis of the vessel-size
over the different VOI for each patient is presented in Table 1. Healthy tissue
values were observed to be in accordance with the literature13, whereas for tumour
regions the vessel-size values were shown to be lower. In our approach, the
tumour volume was defined based on the metabolic active area of FET PET, the
gold standard for tumour extent, which has not been performed in previous
studies11,13. Moreover, it was shown in the literature that there are some
discrepancies between PWI metrics and FET PET14, which might reduce the VSI
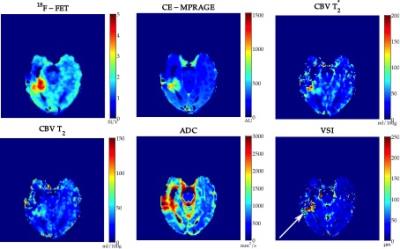
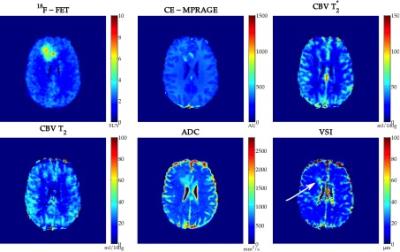
values in the tumour defined by PET. Figures 3 and 4 present the obtained
metrics for two patients. One can observe that CBV, VSI and PET data provide
complimentary information that could lead to deeper understanding of tumour heterogeneity
and could improve treatment monitoring.Conclusion
A method to obtain whole brain vasculature information is proposed by combining SMS and multi-echo EPIK. This method was further tested in tumour patients in the framework of MR-PETAcknowledgements
The authors thank Silke Frensch, Suzanne Schaden, Konny Frey, Dr. Gabriel Stoffels and Dr. Christian Filss for the help with the patient measurements.References
1. Schmiedeskamp H, Straka M, Newbould RD, et al. Combined Spin-And Gradient-Echo Perfusion-Weighted Imaging. Magn Reson Med. 2012;68(1):30-40.
2. Barth M, Breuer F, Koopmans PJ, et al. Simultaneous multislice (SMS) imaging techniques. Magn Reson Med. 2016;75(1):63-81.
3. Eichner C, Jafari-Khouzani K, Cauley S, et al. Slice Accelerated Gradient-Echo Spin-Echo Dynamic Susceptibility Contrast Imaging with Blipped CAIPI for Increased Slice Coverage. Magn Reson Med. 2014;72(3):770-778.
4. Vonken EJ, van Osch MJ, Bakker CJ, et al. Measurement of cerebral perfusion with dual-echo multi-slice quantitative dynamic susceptibility contrast MRI. J Magn Reson Imaging. 1999;10(2):109-117
5. Zaitsev M, Zilles K, Shah NJ. Shared k-space echo planar imaging with keyhole. Magn Reson Med. 2001;45(1):109-117.
6. Zaitsev M, D'Arcy J, Collins DJ, et al. Dual-contrast echo planar imaging with keyhole: application to dynamic contrast-enhanced perfusion studies. Phys Med Biol. 2005;50(19):4491-4505.
7. Yun SD, Reske M, Vahedipour K, et al. Parallel imaging acceleration of EPIK for reduced image distortions in fMRI. Neuroimage. 2013;73:135-143.
8. Setsompop K, Gososki B, Polimeni J, et al. Blipped-Controlled Aliasing in Parallel Imaging (blipped-CAIPI) for simultaneous multi-slice EPI with reduced g-factor penalty. Magn Reson Med. 2012; 65(5):1210-1224
9. Herzog H, Langen KJ, Weirich C, et al. High resolution BrainPET combined with simultaneous MRI. Nuklearmedizin. 2011;50(2):74-82.
10. Stokes AM, Quarles CC. A simplified spin and gradient echo approach for brain tumor perfusion imaging. Magn Reson Med. 2015;75(1):356-362.
11. Kiselev VG, Strecker R, Ziyeh S, et al. Vessel size imaging in humans. Magn Reson Med. 2005;53(3):553-563.
12. Pauleit D, Floeth F, Hamacher K, et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128:678-687.
13. Hsu YY, Yang WS, Lim KE, et al. Vessel size imaging using dual contrast agent injections. J Magn Reson Imaging 2009; 30(5):1078-84.
14. Filss CP, Galldiks N, Stoffels G, et al. Comparison of 18F-FET PET and Perfusion-Weighted MR Imaging: A PET/MR Imaging Hybrid Study in Patients with Brain Tumors. J Nucl Med. 2014;55(4):540-545.