0201
CEST Signals of Lipids1Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Department of High-field Magnetic Resonance, Max-Planck-Institute for Biological Cybernetics, Tübingen, Germany, 3Institute of Biostructure and Bioimaging, Consiglio Nazionale delle Ricerche (CNR), Turin, Italy, 4Department of Molecular Biotechnology and Health Sciences, University of Turin, Turin, Italy
Synopsis
In this study, lipids were identified to be an important contributor to the upfield chemical exchange saturation transfer (CEST) signals of brain tissue. This finding can explain the pronounced CEST image contrast between gray and white matter observed in healthy volunteers.
Purpose
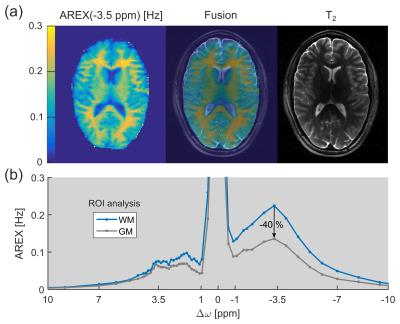
The relayed nuclear Overhauser effect (rNOE) chemical exchange saturation transfer (CEST) signal of aliphatic protons (frequency offset Δω ≈ –3.5 ppm upfield from water) is known to originate from mobile macromolecules, such as proteins/peptides and lipids.1 While rNOE signals of proteins and their dependence on various tissue properties were studied intensively in the past few years, the existence of lipid CEST signals remained to be clarified. In this study, for the first time upfield rNOE signals of brain lipids were experimentally confirmed. These signals can explain the pronounced upfield rNOE contrast between gray (GM) and white matter (WM) observed in healthy brain tissue (Fig. 1).Materials and Methods
Protein-free brain lipids were extracted from mouse brain tissue with tetrahydrofuran, filtered and lyophilized for liposome preparation (size 120 nm) throughout sonication at a concentration of 1 %(w/v). In addition, a protein model solution was studied containing 2.5 %(w/v) bovine serum albumin (BSA). pH of both samples was adjusted to 7.
Isolated CEST signals were calculated via the apparent exchange dependent relaxation2 $$$AREX = \frac{1}{T_{1}} \cdot (\frac{1}{Z} - \frac{1}{Z_{ref}})$$$ employing a bi-Lorentzian fit of the direct water saturation and the semi-solid magnetization transfer (ssMT) as the reference value Zref.
In vivo CEST imaging was performed on a 7 T whole body MR tomograph (MAGNETOM 7 T, Siemens Healthineers, Germany) using a 2D GRE sequence. Pre-saturation was achieved by a train of 150 Gaussian-shaped RF pulses (tpulse = 15 ms, tdelay = 10 ms, tsat = 3.75 s) with a mean amplitude of B1,mean = 0.6 µT. AREX was corrected for B0 and B1-inhomogeneities.
In vitro CEST spectroscopy was performed on a 14.1 T NMR spectrometer (Bruker, Germany) using a continuous wave pre-saturation (tsat = 12 s) with amplitude B1. Z-spectra were obtained for various B1 values ranging from 0.25 to 2.0 µT.
Results
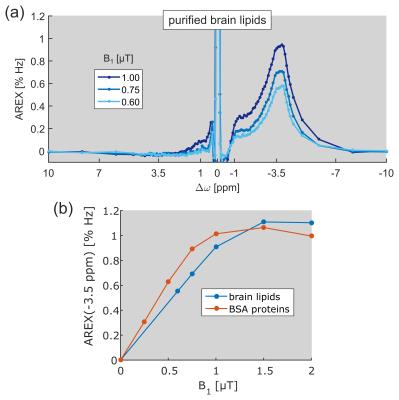
Region of interest (ROI) analysis of GM and WM of a healthy volunteer yielded a mean difference of the upfield rNOE signal of approximately –40% (Fig. 1b). This signal difference is extraordinarily large in comparison to CEST signal variations at the opposite side of the water (Δω > 0). At the same frequency offset, Δω ≈ –3.5 ppm, a strong CEST signal can be resolved in the AREX spectra of purified brain lipids (Fig. 2a). The spectral position of this newly-discovered CEST signal matches with the resonance frequencies of aliphatic protons in lipids. The build-up of the lipid CEST signal as a function of B1 complies with the rNOE signal characteristics of BSA proteins (Fig. 2b). Interestingly, no CEST signals could be observed in the measurement of synthetic liposomes (data not shown).Discussion
A pronounced upfield rNOE contrast between GM and WM was also reported in previous studies.3,4 This finding cannot be explained solely by a different concentration/composition of proteins in GM and WM, because otherwise comparable large signal differences must also be observable for the protein CEST signal of amide protons at +3.5 ppm (Fig. 1b). However, as WM is known to consist of large amounts of lipids in the myelin sheath, a possible explanation for the observed upfield rNOE contrast is the existence of CEST signals of lipids.
Indeed, measurements of purified brain lipids in this study revealed substantial rNOE signals upfield from water (Fig. 2a). The occurrence of CEST signals solely in the frequency range of aliphatic protons proves the assignment to lipid species. Because aliphatic protons are covalently bound and are therefore non-exchanging, a magnetization transfer from lipid to water protons preferably occurs via rNOE pathways. The built-up of the lipid CEST signal as a function of B1 similar to that of rNOE signals of proteins supports this conception (Fig. 2b). Furthermore, the amplitude of rNOE signals is comparable to that of proteins, allowing to conclude that the pronounced upfield rNOE contrast between GM and WM most likely originates from differences in lipid content.
Conclusion
The existence of upfield rNOE signals of lipids was experimentally verified by investigation of purified brain lipid samples. Consequently, caution is needed when assigning the upfield rNOE signal to proteins, especially in the case of tissues with high lipid content such as WM.Acknowledgements
No acknowledgement found.References
1. van Zijl PCM, Zhou J, Mori N, et al. Mechanism of Magnetization Transfer During On-Resonance Water Saturation. A New Approach to Detect Mobile Proteins, Peptides, and Lipids. Magn Reson Med. 2003;49:440-449.
2. Zaiss M, Xu J, Goerke S, et al. Inverse Z-spectrum analysis for spillover-, MT-, and T1-corrected steady-state pulsed CEST-MRI – application to pH-weighted MRI of acute stroke. NMR Biomed. 2014;27(3):240-252.
3. Jones CK, Huang A, Xu Jiadi, et al. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7 T. NeuroImage. 2013;77:114-124.
4. Zaiss M, Windschuh J, Paech D, et al. Relaxation-compensated CEST-MRI of the human brain at 7 T: Unbiased insight into NOE and amide signal changes in human glioblastoma. NeuroImage. 2015;112:180-188.
Figures