0186
Investigating structural brain change with heart failure using voxel-based morphometry1Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 2Herzzentrum Leipzig, Leipzig, Germany, 3Leipzig Research Center for Civilization Diseases (LIFE), Leipzig, Germany, 4Department of Neurology, Center of Neurology and Neurosurgery, University Hospital Frankfurt, Frankfurt, Germany, 5Institute of Laboratory Medicine, University Hospital Leipzig, Leipzig, Germany, 6Clinic for Cognitive Neurology, University Hospital Leipzig, Leipzig, Germany
Synopsis
Heart failure is a multifactorial disease including a reduced pump efficiency leading to an insufficient oxygen supply for all body organs. However, the consequence of heart failure to brain structure is an important issue that needs further investigation. We used structural MRI with voxel-based morphometry to investigate a relationship between gray matter density and heart failure using ejection fraction and N-terminal prohormone of brain natriuretic peptide as markers for disease severity. These markers were found to be associated with decreased gray matter density in orbitofrontal and hippocampal brain regions indicating local gray matter abnormalities in these regions with heart failure.
Introduction
Heart failure is a common condition of heart damage leading to a decreased blood flow due to a reduced pump efficiency of the heart muscle. A consequence can be insufficient oxygen supply to the organism including the brain. While heart failure clearly shows neurological symptoms, such as fatigue, nausea and dizziness, the consequences to brain structure are not well understood. Few studies show regional gray matter (GM) decrease related to heart failure1,2,3, however, the underlying mechanisms leading to the observed brain changes remain unclear.
Recent work showed both hippocampal GM loss4 and blood flow abnormality5 suggesting a link between brain damage and decreased blood flow due to a decreased heart pumping efficiency. To further investigate the relationship between structural brain change and impairment of heart function and its consequences to blood circulation, we studied the potential correlation between gray matter density (GMD) and ejection fraction (EF) using voxel-based morphometry (VBM).
Methods
A potential relationship between heart failure markers and brain structure was investigated in a group of 50 patients (12 female, mean age=53.9 y, std=5.4 y). All patients received a coronary stent placed during a percutaneous coronary intervention. EF (mean=56.4%, std=11.8%) and N-terminal prohormone of brain natriuretic peptide (NT-proBNP, mean=220.6 pg/mL, std=306.0 pg/mL) were used as markers for screening, diagnosis and prognosis of congestive heart failure. Structural T1-weighted MP-RAGE brain images were acquired on a 3T Verio scanner (Siemens, Erlangen, Germany) with a 32-channel head coil using 176 sagittal slices, a field of view of 240×256 mm2, and a nominal resolution of 1×1×1 mm3. Imaging parameters were chosen according to the ADNI protocol (TR=2300 ms, TE=3 ms, TI=900 ms).
VBM was performed using GMD images computed with the Computational Anatomy Toolbox (CAT) in combination with SPM12 and Matlab 8.6. Statistical analysis was performed with a voxel threshold of p<0.005 using the general linear model to test for potential correlations between GMD and both markers of heart failure (EF, NT-proBNP) including age and total intracranial volumes as covariates. To correct for multiple comparisons, significant clusters were obtained using a family-wise error corrected cluster threshold of p<0.05 (k>1000 voxels).
Results
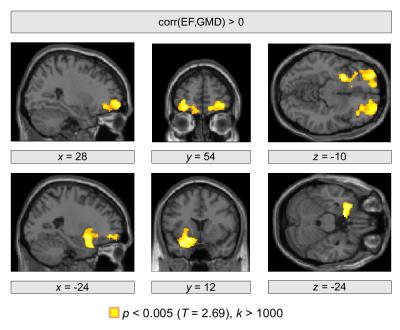
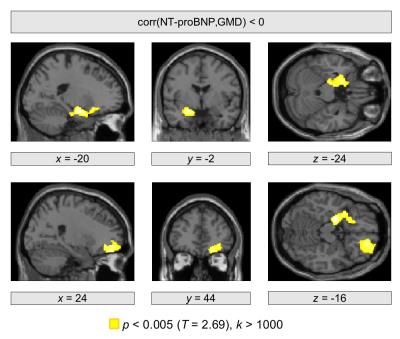
We obtained a significant negative correlation between EF and NT-proBNP (r=−0.43 , p=0.0018). Using structural brain images, significant correlations between brain structure and both heart failure markers were revealed in the frontal cortex as well as in subcortical regions in the left hemisphere. In particular, a positive correlation between EF and GMD was observed bilaterally in the orbitofrontal cortex (p=0.020, p=0.013; Figure 1). A further significant cluster was obtained in the left ventral striatum (p=0.004). Significant negative correlations between NT-proBNP and GMD were observed in the right orbitofrontal cortex (p=0.047) and in the left hippocampus (p=0.017; Figure 2).Conclusions
We obtained significant correlations between brain structure and markers of heart failure including EF and NT-proBNP. A diminished GMD was found with decreased EF and increased NT-proBNP in orbitofrontal regions, which is in line with previous work showing a reduced cortical thickness in heart failure patients in these cortical regions6. We note that the orbitofrontal cortex plays a substantial role in blood pressure regulation7, which might link reduced EF, potentially diminished blood flow, and structural brain change.
We also found increased NT-proBNP with a diminished GMD in the hippocampus in our patient cohort. Structural abnormalities in the hippocampus were previously shown in rats with heart failure using VBM and probabilistic maps of the Wistar rat brain4. In line with these observations, histological analysis revealed a decreased neurogenesis together with an increased number of astrocytes in the ventral hippocampus of rats suffering from heart failure compared with sham rats4. Thus, the relationship between NT-proBNP concentrations and GMD might reflect brain injury due to changes in hippocampal blood flow in HF5.
Acknowledgements
No acknowledgement found.References
1. Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper R. Regional brain gray matter loss in heart failure. J. Appl. Physiol. 2003;95(2):677-684. doi: 10.1152/japplphysiol.00101.2003
2. Menteer J, Macey PM, Woo MA, Panigrahy A, Harper RM. Central Nervous System Changes in Pediatric Heart Failure: A Volumetric Study. Pediatric Cardiology 2010;31(7): 969-976. doi: 10.1007/s00246-010-9730-9
3. Almeida OP, Garrido GJ, Beer C, Lautenschlager NT, Arnolda L, Flicker L. Cognitive and brain changes associated with ischaemic heart disease and heart failure. European Heart Journal 2012;33(14):1769-1776. doi: 10.1093/eurheartj/ehr467
4. Suzuki H, Sumiyoshi A, Matsumoto Y, Duffy BA, Yoshikawa T, Lythgoe MF, Yanai K, Taki Y, Kawashima R, Shimokawa H. Structural abnormality of the hippocampus associated with depressive symptoms in heart failure rats. Neuroimage 2015;105:84-92. doi: 10.1016/j.neuroimage.2014.10.040
5. Suzuki H, Matsumoto Y, Ota H, Sugimura K, Takahashi J, Ito K, Miyata S, Furukawa K, Arai H, Fukumoto Y, Taki Y, Shimokawa H. Hippocampal Blood Flow Abnormality Associated With Depressive Symptoms and Cognitive Impairment in Patients With Chronic Heart Failure. Circ J. 2016;80(8):1773-1780. doi: 10.1253/circj.CJ-16-0367
6. Kumar R, Yadav SK, Palomares JA, Park B, Joshi SH, Ogren JA, Macey PM, Fonarow GC, Harper RM, Woo MA. Reduced Regional Brain Cortical Thickness in Patients with Heart Failure. PLoS ONE 2015;10(5):e0126595. doi:10.1371/journal.pone.0126595
7. Wong SW, Massé N, Kimmerly DS, Menon RS, Shoemaker JK. Ventral medial prefrontal cortex and cardiovagal control in conscious humans. Neuroimage 2007;35(2):698-708. doi: 10.1016/j.neuroimage.2006.12.027
Figures