0184
Novel Design of a 3D-Printed Anthropomorphic Brain Phantom for Segmentation Validation in Magnetic Resonance Imaging1Department of Biomedical Engineering, University Basel, Basel, Switzerland, 2Medical Image Analysis Center (MIAC) AG, Basel, Switzerland, 3Department of Radiology, University Hospital Basel, Basel, Switzerland, 4Department of Neurology, University Hospital Basel, Basel, Switzerland
Synopsis
Precise brain phantoms are important for evaluating the quality of segmentation tools for brain MRI. Here we suggested the construction of a 3D physical brain phantom as gold standard to validate the performance of those tools. Folding patterns of grey and white matter compartments were replicated using 3D-printed models from a real structural brain scan. T1 and T2 intensities of these brain regions in a 3 Tesla MRI were mimicked by a 0.6% agar mixture containing the appropriate concentrations of the paramagnetic compounds Ferumoxide and Manganese chloride. With its 3D-printed brain-like design, the phantom showed to be a promising alternative to existing methods for MRI segmentation validation.
Purpose
Knowledge of the exact spatial distribution and precise volumes of brain
tissues in images acquired by magnetic resonance imaging (MRI) are important
when evaluating segmentation quality. However, to date, tissue segmentation
algorithms are lacking of a ground truth for efficient and reliable validation
and comparison 1. The most popular validation methods include the use of software simulations
2,3 and phantoms 4,5. No phantom has ever been constructed that at the same time mimics
relaxation times and folding patterns of different brain compartments. Accordingly,
the aim of this study is to develop the first 3D-printed brain-like phantom to
verify grey matter (GM) and white matter (WM) segmentation in MRI.
Methods
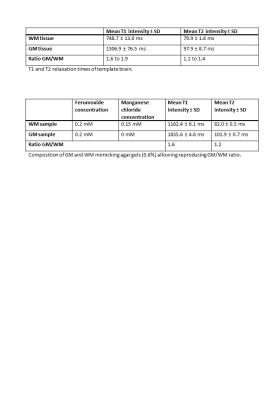
The study was divided in three stages. Initially, MRI scans of a healthy subject were performed to derive a ground truth for brain T1 and T2 values. T1 relaxation maps were acquired at a clinical 3 Tesla (T) Whole-Body MRI Scanner with a 20-channel head coil using an inversion recovery spin echo sequence (resolution = 1x1x5 mm3, TR = 6000 ms, TE = 12 ms, TI = 30/50/100/200/500/750/1000/2000 ms). T2 relaxation maps were acquired using a multi-echo spin echo sequence (resolution = 1x1x5 mm3, TR = 3000 ms, TE = 13.2 - 422.4 ms, echo spacing 13.2 ms). Average T1 and T2 intensities within regions of interest (ROIs) for WM (2 ROIs of dimensions 3x3 mm2) and GM (4 ROIs of dimensions 3x3 mm2) were computed. The ratio derived from average intensities of WM and the ones of GM was then mimicked by testing different concentrations of the two MRI contrast agents Manganese Chloride (MnCl2; concentrations = 0 to 0.25 mM) and Ferumoxide (concentrations = 0 to 10 mM) in a 0.6% agar gel.
At a second stage, anatomic scans were performed to define a model for the construction of the brain scaffold. A whole-brain isotropic MPRAGE scan (resolution = 1x1x1 mm3, TR/TI/TE = 1570/900/2.48 ms, α = 8, Figure 1) of the same subject was automatically segmented by Freesurfer (Harvard University, Cambridge, Massachusetts, USA), allowing the identification of the pial surface and the GM/WM interface. These surfaces were used as template for the phantom shape and, thus, converted to two stereolithography files. The corresponding mesh density was reduced by 50% of the initial triangle count, the surfaces slightly smoothed, and the GM/WM boundary surface was decreased by 40 mm of its maximal width in the sagittal plane (Meshmixer 2.9, Autodesk, Inc., San Rafael, California, USA). Only the surfaces of the left hemisphere were then 3D-printed using polylactic acid (PLA) thermoplastic (Figure 2). To create flexible molds, easily allowing the detachment of the paramagnetic gels, the 3D-printed surfaces were covered by a brushable platinum-cure silicone rubber with a shore A hardness of 30 (Figure 3).
At the last construction stage, the phantom was assembled as follows. The GM/WM interface mold was filled with the WM solution, the mold was removed, the pial surface mold was placed on top of the WM gel and the GM solution was injected into the gap between WM gel and outer silicone cast. Finally, for the evaluation of the phantom, the same inversion recovery spin echo, multi-echo spin echo, and MPRAGE sequences were used to verify the structural accuracy as well as the T1 and T2 intensities of the two phantom compartments.
Results
All the steps previously described were completed and the brain phantom was successfully constructed. The ratio between average GM and WM intensities calculated in the healthy (Table 1) was achieved through the gel compositions depicted in Table 2. Moreover, the constructing procedure allowed to reproduce folding patterns typical of GM and WM (Figure 4).Discussion
The 3D printed anthropomorphic phantom showed to be a promising alternative to MRI phantoms currently adopted for the brain. However, other fundamental steps and adjustments are necessary before the real application. For example, precise T1 and T2 intensities rather than GM/WM ratios will be mimicked, a compartment that mimics the cerebrospinal fluid will be added, and further tests allowing the phantom assembly beyond a more realistic GM volume are needed.Conclusions
This phantom is an important step forward in the creation of a realistic physical brain phantom and in the provision of ground truth for comparing and validating segmentation algorithms in MRI.Acknowledgements
References
1. Despotovic, I., Goossens, B. & Philips, W. MRI Segmentation of the Human Brain: Challenges, Methods, and Applications. Comput. Math. Methods Med. 2015, (2015).
2. Collins, D. L. et al. Digital Brain Phantom. IEEE Trans. Med. Imaging 17, 2005–2007 (2007).
3. IBSR, "The Internet Brain Segmentation Repository," 2013, http://www.nitrc.org/projects/ibsr.
4. Khan, A. F. et al. A novel MRI-compatible brain ventricle phantom for validation of segmentation and volumetry methods. J. Magn. Reson. Imaging 36, 476–82 (2012).
5. Chen, S. J. et al. An anthropomorphic polyvinyl alcohol brain phantom based on Colin27 for use in multimodal imaging. Med. Phys. 554, (2013).
Figures