0183
Brain MR image intensity normalization in the presence of pathology1Image Sciences Institute, University Medical Center Utrecht, Utrecht, Netherlands, 2Department of Epidemiology, University Medical Center Utrecht, Utrecht, Netherlands
Synopsis
Brain MR image intensities do not have a fixed tissue-specific value. Especially in longitudinal studies, where a subjects’ anatomy might change, pathology can arise, scanner software and hardware may be replaced, the resulting image intensities can differ widely. This thwarts subsequent post-processing or image analysis. Various image intensity normalization techniques exist, but are often evaluated on healthy subjects. In this work, we evaluate six normalization techniques on 25 image-pairs (five year interval) of subjects with brain pathology. Traditional methods (e.g. Gaussian and Z-Score) are clearly affected by the presence of pathology and perform less than more recent techniques.
Purpose
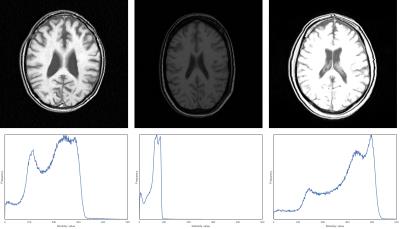
Brain MR imaging provides excellent soft tissue contrast, but unfortunately does not provide tissue specific intensity values. As a result, similar imaging protocols produce different intensities for the same tissue type1,2. An example of such variations is provided in Figure 1. Especially in longitudinal studies, changes to scanner software and hardware are to be expected and can introduce intensity differences.
Intensity normalization techniques aim to reduce the intensity variation of images and are usually applied before subsequent post-processing or image analysis. Most normalization techniques are based on a histogram matching approach1–6. Evaluation of many techniques is done on images of healthy volunteers, whereas the presence of pathology is likely to thwart histogram matching approaches.
This work investigates the performance of intensity normalization techniques in the presence of white matter hyperintensities (WMH) and infarctions. We focus on normalizing intensity differences between image-pairs of the same subjects, acquired at two different time points with a five year interval.
Methods
We purposively selected 25 subjects with varying degrees of WMH and infarcts from the SMART-MR study, who also had a follow-up scan available after five years. Subjects underwent a 1.5T MR acquisition, including a transversal T1-weighted gradient-echo sequence (TR/TE: 235/2 ms; flip angle: 8°; FOV: 230×230 mm; matrix: 256×256×38; voxel size: 0.898×0.898×4.0 mm)7. WMH load on baseline/follow-up images is (mean±sd) 6.76±8.55 ml / 6.94±7.85 ml, respectively. Infarct volume on baseline/follow-up images is 9.93±17.60 ml / 18.71±29.03 ml, respectively.
Six intensity normalization methods were selected for evaluation.
* Gaussian: rescaling intensities by: $$$I_{new} = \frac{I}{SD}$$$, where I is the intensity and SD the standard deviation of all intensities.
* Z-Score: also known as “zero-mean-unit-variance”, rescaling by: $$$I_{new} = \frac{I - \mu}{SD}$$$, where μ is the mean intensity.
* HMmedian: a histogram matching (HM) approach based on three landmarks: s1, s2, and µs. S1 and s2 are the 0th and 99.8th percentile of the histogram2. µs is the mode of all intensity values above the mean intensity, effectively selecting the white matter in the brain (which is the most common structure in a brain slice). These values are piece-wise linear mapped to a standard intensity scale, which is obtained by training and averaging the landmarks on four subjects.
* HMball: this method is essentially the same as HMmedian, except the computation of µs differs3. On the middle slice in the image, the largest connected homogeneous area is determined. This should correspond to the normal appearing white matter and its median intensity is used as µs.
* STI: each image is nonlinearly warped to a reference image1.The reference image intensities are rescaled to 0-100 and binary masks of the grey matter, white matter, and cerebrospinal fluid are available (e.g. from the MNI152 template8). A joint histogram is made for each tissue type and the mode selected as landmark. Images are piece-wise linear mapped between 0-100 (min-max) and the three landmarks.
* MIMECS: an image synthesis technique based on patch replacement9. For each voxel in the image, a 3×3×3 patch is created, compared to patches of a reference images, and then replaced by the intensity of the best match.
To evaluate, we computed the absolute intensity difference between the two images of each subject. In order to do so, all images were aligned with the MNI152 template image. Tissue labels were obtained with an established kNN segmentation10,11 and evaluation was only performed for voxels having equal tissue labels in both images.
Results
Results are summarized in Figure 2. Wilcoxon signed rank tests were applied between the original images and the normalized results. Asterisks below the bar indicate statistically significant differences, having p<0.05. The effect of intensity normalization is clearly visible, especially for HMball, STI, and MIMECS. The Gaussian and Z-Score methods are affected by the presence of WMH in the images.Discussion
All evaluated methods are able to reduce the image intensity differences between follow-up images of subjects. Intensity differences for WM and GM are reduced the most, whereas the original intensities for CSF were already quite similar and improvement was less. Simple normalization strategies, such as Gaussian and Z-Score, are affected by the presence of pathology (WMH) and show the worst performance. This should be taken into account, as Z-Score is a highly popular intensity normalization method. MIMECS shows good performance, but since it utilizes a patch-replacement strategy it should be used carefully, as it can replace/remove original image features.Conclusion
Intensity normalization is important to consider and the techniques HMball, STI, and MIMECS show good performance in a longitudinal study on images with pathology.Acknowledgements
No acknowledgement found.References
1. Robitaille, N. et al. Tissue-based MRI intensity standardization: application to multicentric datasets. Int. J. Biomed. Imaging 2012, 347120 (2012).
2. Nyúl, L. G., Udupa, J. K. & Zhang, X. New Variants of a Method of MRI Scale Standardization. IEEE Trans. Med. Imaging 19, (2000).
3. Madabhushi, A. & Udupa, J. K. New methods of MR image intensity standardization via generalized scale. Med. Phys. 33, 3426–3434 (2006).
4. Nyúl, L. G. & Udupa, J. K. On standardizing the MR image intensity scale. Magn. Reson. Med. 42, 1072–81 (1999).
5. Ellingson, B. M. et al. Comparison between intensity normalization techniques for dynamic susceptibility contrast (DSC)-MRI estimates of cerebral blood volume (CBV) in human gliomas. J. Magn. Reson. Imaging 35, 1472–1477 (2012).
6. Christensen, J. D. Normalization of brain magnetic resonance images using histogram even-order derivative analysis. Magn. Reson. Imaging 21, 817–820 (2003).
7. Geerlings, M. I. et al. Brain volumes and cerebrovascular lesions on MRI in patients with atherosclerotic disease. The SMART-MR study. Atherosclerosis 210, 130–136 (2010).
8. Fonov, V. et al. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage 54, 313–27 (2011).
9. Roy, S., Carass, A. & Prince, J. L. Patch based intensity normalization of brain MR images. in 2013 IEEE 10th International Symposium on Biomedical Imaging 2013, 342–345 (IEEE, 2013).
10. Anbeek, P., Vincken, K. L., van Osch, M. J. P., Bisschops, R. H. C. & van der Grond, J. Probabilistic segmentation of white matter lesions in MR imaging. Neuroimage 21, 1037–44 (2004).
11. Anbeek, P., Vincken, K. L., van Bochove, G. S., van Osch, M. J. P. & van der Grond, J. Probabilistic segmentation of brain tissue in MR imaging. Neuroimage 27, 795–804 (2005).
Figures