0182
Quality Assurance of Neuro-MRI within Multicenter Clinical Trials1Wright Center of Innovation in Biomedical Imaging, The Ohio State University, Columbus, OH, United States
Synopsis
Volumetric analysis and quantification especially of dynamic MR sequences is increasingly invaluable for assessing treatment response and time to progression in the majority of clinical trials focusing on neuro-oncology. These innovative therapeutic trials rely heavily on consistent image acquisitions even in multi-center Phase 2/3 trials. We have developed a highly structured DICOM tag based, parameter driven, semi-automated QC approach that readily enables visualization of acquisition inconsistencies using a heat-mapping spectrum. As the imaging core laboratory for several NCI-NCTN clinical trials, we expanded the QC methodology to also enable constructive feedback education / training to help improve the quality of Neuro-MRI submissions.
Purpose
With the increased use of quantification techniques and imaging based response assessment, standardization of imaging acquisitions in multicenter Phase 2/3 clinical trials is critical. Our team serves as an imaging core laboratory within NCI’s Imaging and Radiation Oncology Core (IROC) cooperative, and is involved in several such trials that include Neuro-MRI. We provide several services such as site credentialing, quality assurance, site feedback, image banking and radiological review for response assessment. We have analyzed the variability of MRI acquisitions, specifically in neuro-imaging trials and hope to raise awareness as well as explore innovative solutions to improve quality and consistency.Methods
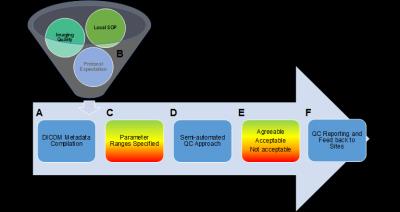
We have previously developed for an abdominal MR imaging trial, a highly structured DICOM based, parameter driven, semi-automated QC approach that helps in identifying “hot-spots” or problem areas of protocol non-conformance through a parameter driven visualization of variations (Figure 1). We now adapted this approach for Neuro-MRI especially as International guidelines for Neuro-MRI have been evolving. Due to the interdependence of MR parameters, the overall compliance of each sequence is assessed using a weighted scoring system, with score assignments proportional to the impact of parameter influence on imaging quality as identified by the imaging committee.
For this study, we analyzed MRI examinations submitted for an ongoing Phase 2/3 Glioblastoma trial, performed on a sample of 30 patients, from 25 different institutions, over 4 time-points (Baseline and 3 follow-up scans, including progression). Specifically, we focused on the DICOM metadata distribution of dynamic MR sequences, whose quantitative assessment as predictors for time to progression was one of the clinical goals of this demonstration trial.
Results
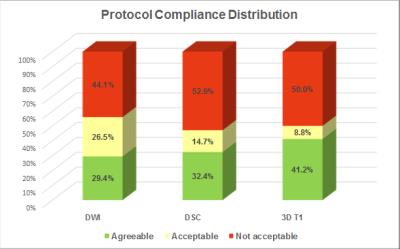
The above described DICOM based QC approach was used to assess the quantification rating of the following three key sequences: 1) Diffusion Weighted Imaging (DWI), 2) 3D T1-weighted isotropic acquisition with inversion preparation (before and after contrast administration), and Dynamic Susceptibility Contrast (DSC) imaging (Figure 2). A weighted score was then assigned to each examination. Surprisingly less 30% of the submitted examinations were completely within the protocol expectations. In nearly half of the exams submitted, the reliable use of DSC and DWI metrics would be challenging.
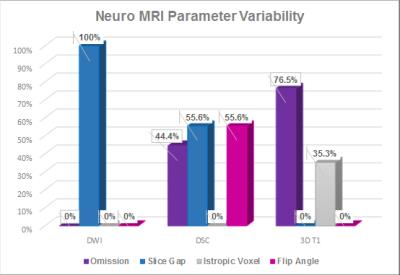
A parametric breakdown analysis of each of these sequences helps in locating factors contributing to these non-conformant images. For example, almost a 100% of the DWI submissions identified as Red could have been fixed if acquired without slice gaps (Figure 3). Similarly, isotropic voxel volume and consistency of imaging both before and after contrast administration are critical for a T1-weighted subtraction map analysis of tumor to accurately quantify enhancing tumor burden. Inconsistency of parameters pre and post contrast (2D vs 3D, or omission of pre-contrast sequence) was found to be the biggest contributing factor. In addition, inconsistencies between baseline and follow-up images were observed in more than 75% of the patients analyzed.
This analytical QC approach enabled us to provide sites with specific recommendations to improve protocol conformance, as well as report study compliance to the imaging committee in great detail, on a regular basis. Such feedback mechanism was extremely valuable in identifying and addressing some ambiguity in protocol language including timely submission of images to IROC Ohio for increased impact of feedback and communication. At the time of this abstract, an update to the protocol addressing these concerns was broadcast to the participating institutions.
Discussion
MRI metadata is readily accessible as demonstrated by this innovative quality assurance methodology, highlighting significant variations between established standards within an institutional setting, to the reality of true variability in image acquisition. The developed approach readily enables feedback and training / learning opportunities through an effective and efficient visual assessment of overall and/or specific performance characteristics.
Standardization of image acquisition should, therefore, be a priority to raise the quality of local practices in keeping with the consensus recommendations, eventually leading to an increased submission of viable data for quantitative and qualitative analysis in multicenter clinical trial settings.
Conclusion
While phantom based performance assessment characterizes the ability of the MRI system to meet industry or expected clinical trial system standards, today, the most quality impacting variability occurs in the extensive variability of MRI acquisition parameters which are commonly not readily recognized and mapped. Innovative quality assurance methodologies such as developed and presented for the performance of Neuro-MRI within therapeutic multicenter clinical trials are indispensable to identify and address inconsistencies to ensure the desired quality of imaging readouts.Acknowledgements
The funding of IROC Ohio through the NIH grant 5U24CA180803 is gratefully acknowledged as well as the infrastructure support by ODSA TECH 09-028.References
1. FitzGerald TJ, Bishop-Jodin M, et al. Imaging and Data Acquisition in Clinical Trials for Radiation Therapy. Int J Radiat Oncol Biol Phys. 2015.
2. Ellingson BE, Bendszus M, et al. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol. 2015.
Figures