0179
Improving angular resolution in multi-shot turbo spin-echo diffusion imaging using rotating single-shot acquisition (RoSA)1Indiana University, Indianapolis, IN, United States, 2University of College London
Synopsis
The rotating single-shot acquisition (RoSA) technique is proposed to accelerate multi-shot diffusion imaging acquisitions by acquiring one shot per diffusion direction. The RoSA approach utilizes similarity existing in diffusion-weighted contrast for image reconstruction. It has been successfully implemented with echo planar imaging (EPI). In this study, we use the RoSA approach to improve turbo spin-echo (TSE) based multi-shot sequences. In particular, we will demonstrate that with the same acquisition time, RoSA increases the diffusion angular sampling resolution by 3-fold compared to a Turboprop sequence.
Introduction
Multi-shot techniques are commonly used for diffusion MRI to achieve high spatial resolution. In this category, Turboprop1, a faster version of PROPELLER2, is a self-navigated technique robust to patient motion. Furthermore its distortion-free feature makes it desirable in many clinical applications. However, due to its multi-shot nature, the prolonged acquisition time limits its research and clinical uses, especially as current developments in diffusion imaging favor higher angular resolution3-4. The recently proposed rotating single-shot acquisition (RoSA) technique aims to accelerate multi-shot diffusion-weighted (DW) acquisitions by acquiring one shot per DW direction, and the similarity feature of DW images are utilized in its composite reconstruction3. It has been successfully implemented with echo planar imaging (EPI) readouts5, and is capable of improving turbo spin-echo (TSE) based multi-shot sequences. In this work, we used simulation of human brain data to demonstrate that with the same acquisition time, RoSA increases the diffusion angular resolution to 3-fold. The results were evaluated with diffusion tensor imaging (DTI), neurite orientation distribution and density imaging (NODDI), and fiber orientation distribution function (FOD).Methods
A set of brain DW images (i.e., ground truth) was simulated using the framework proposed in ref 6, with 1mm isotropic resolution, 1 b0, 90 diffusion directions at b=1000s/mm2 and 90 directions at b=2000s/mm2.
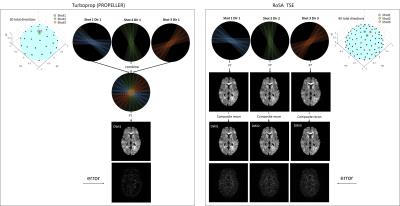
Turboprop simulation: Turboprop dataset was obtained by cropping the k-space of ground-truth DW images to simulate the real acquisition process without the consideration of T2 decay. The parameters used were: a blade size of 256×32, 4 blades per shot, 12 blades per image, thus 3 shots per diffusion direction (Figure 1 left, 1st row). To reconstruct one direction DW image, k-space blades from 3 shots were combined and density corrected to produce one DW image (Figure 1 left, 2nd and 3rd row).
RoSA simulation: RoSA was simulated in a similar way with matched acquisition parameters, except that 1 shot was acquired per DW direction (Figure 1, right). Thus, for 90 shots, 30 directions were obtained in Turboprop, whereas 90 directions were obtained in RoSA.
RoSA reconstruction: Upon reconstructing the i’th direction DW image, its neighborhood directions in the q-space sphere were searched and the closest directions with different k-space coverage patterns were selected. These blades were combined with the composite reconstruction to obtain the high-resolution DW image, as described in 5,7. The right panel in Figure 1 shows DW images (2nd row) after direct Fourier Transformation of undersampled k-space in the 1st row, and reconstructed high-resolution RoSA DW images in the 3rd row.
With the same number of shots, Turboprop obtained 30 diffusion directions for both b-values, whereas the RoSA scheme obtained 90 directions. The inner-shell was fitted with DTI, outer-shell was fitted with FOD using lmax=6 in MRtrix38, and both shells were fitted with NODDI. Residual errors of diffusion metrics were examined by comparing to the ground truth. To evaluate the FOD results, angular correlation coefficients9 were computed for each voxel between each method and the ground truth.
Results
The error maps of reconstructed DW images are displayed in Figure 1 (bottom row). As expected, RoSA demonstrated higher error than Turboprop due to a 3-fold undersampling in k-space. Despite of this, matching DW contrast can be appreciated between RoSA and Turboprop (3rd row).
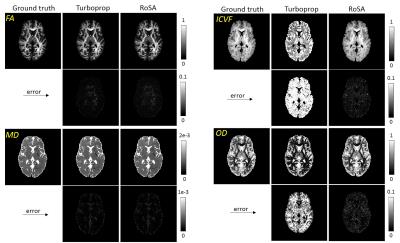
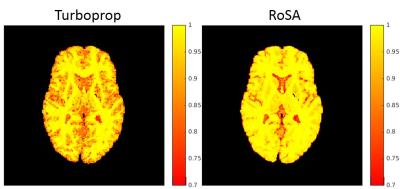
For DTI, both Turboprop and RoSA had minimum image errors in fractional anisotropy (FA) and mean diffusivity (MD) (Figure 2 left). NODDI metrics, however, had dramatic difference between the two methods, with Turboprop showing 62 times higher error in intra-neurite volume fraction (ICVF) than RoSA (0.25 vs 0.004) and 15 times higher error in orientation dispersion index (OD) than RoSA (0.055 vs 0.004). Figure 3 shows angular correlation coefficients maps of FOD where 1 indicates perfect correlation. RoSA demonstrated near perfect correlations with the ground truth in white and most grey matter regions. Turboprop had high angular correlations in white matter, but was quickly degraded when transitioning to grey matter.
Discussion
We demonstrated that RoSA scheme achieved 3 times higher angular resolution than Turboprop, allowing reliable DTI and significantly improved NODDI and FOD estimation. The high angular resolution is achieved by trading off k-space coverage for q-space sampling resolution, and the missing k-space data is later reconstructed by utilizing similarities in neighboring DW images. Such improvement would open up new and exciting opportunities for research and clinical studies in which diffusion angular sampling resolution is compromised by long scan time in multi-shot sequences. Similar to Turboprop, RoSA TSE is self-navigated, distortion-free and robust to motion artifact. Further work will focus on validation with the human data.
Acknowledgements
No acknowledgement found.References
1. Pipe JG, Zwart N. Turboprop: improved PROPELLER imaging. Magn Reson Med 2006; 55: 380-385.
2. Pipe JG, Farthing VG, Forbes KP. Multi-shot diffusion-weighted FSE using PROPELLER MRI. Magn Reson Med. 2002 Jan;47(1):42-52.
3. Tournier JD, Yeh CH, Calamante F, Cho KH, Connelly A, Lin CP. Resolving crossing fibres using constrained spherical deconvolution: validation using diffusion-weighted imaging phantom data. Neuroimage. 2008 Aug 15;42(2):617-25.
4. Zhang, H., T. Schneider, C.A. Wheeler-Kingshott, and D.C. Alexander, NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage, 2012. 61(4): p. 1000-16.
5. Wen Q, Kodiweera C, Wu YC. “Windowed” Composite Reconstruction Improves Rotating Short-Axis High-Resolution DWI (RSA-DWI) in both Simulation and Human data. Proceedings of the 24th Annual Meeting ISMRM, Singapore, 2016; 516.
6. Graham MS, Drobnjak I, Zhang H. Realistic simulation of artefacts in diffusion MRI for validating post-processing correction techniques. Neuroimage. 2016 Jan 15;125:1079-94.
7. Mistretta CA, Wieben O, Velikina J, Block W, Perry J, Wu Y, Johnson K, Wu Y. Highly constrained backprojection for time-resolved MRI. Magn Reson Med. 2006. Jan;55(1):30-40.
8. Tournier JD, Calamante F, Gadian DG, Connelly A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage. 2004 Nov;23(3):1176-85.
9. Anderson AW. Measurement of fiber orientation distributions using high angular resolution diffusion imaging. Magn Reson Med. 2005 Nov;54(5):1194-206.
Figures