0174
High resolution whole brain diffusion MRI at 7 Tesla using RF parallel transmission1Radiology, Medical School, University of Minnesota, Minneapolis, MN, United States, 2Center for Imaging of Neurodegenerative Diseases, VA Healthcare System, San Francisco, CA, United States, 3Physikalisch-Technische Bundesanstalt, Berlin, Germany
Synopsis
A major component of the Human Connectome Project (HCP) in the WU-Minn consortium is multiband (MB)-accelerated whole-brain diffusion MRI (dMRI) at both 3T and 7T. Although having some advantages over 3T dMRI in inferring connectivity, the 7T acquisition suffers from RF nonuniformity and is limited to MB2 acceleration because of SAR. Here, we demonstrate the utility of RF parallel transmission (pTx) for 7T HCP-type dMRI with ~1-mm isotropic resolution. Our results demonstrate that pTx can significantly improve RF uniformity across the entire brain and enable higher slice acceleration relative to single transmit configurations, thereby holding great potential for acquiring high quality, high resolution and high efficiency dMRI data.
Purpose
To investigate how RF parallel transmission (pTx) can be used to improve the image quality when acquiring Human Connectome Project (HCP)-type whole-brain diffusion MRI (dMRI) at 7 Tesla (7T) with ~1-mm isotropic resolutions.Methods
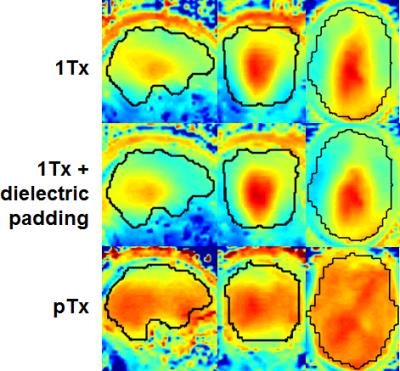
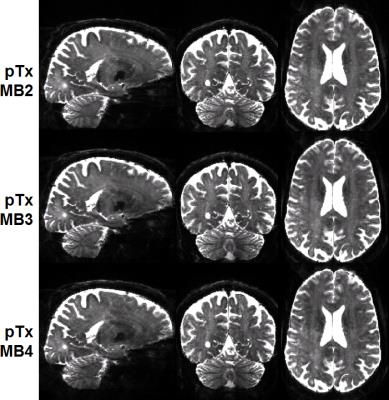
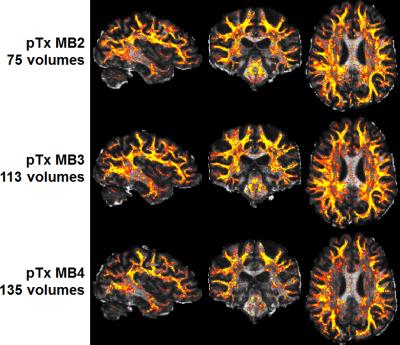
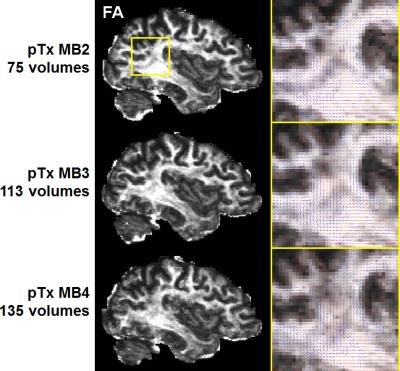
Human studies were conducted on a 7T MR scanner (Siemens, Erlangen, Germany), which can be operated in a single transmit (1Tx) or pTx mode. Healthy subjects who signed a consent form were scanned. Each subject was scanned first in the pTx mode using the Nova 8 transmit 32 receive (8Tx/32Rx) head coil (Nova Medical, Inc., MA, USA) and then in the 1Tx mode using the Nova 1Tx/32Rx head coil. For pTx, the coil was used in the “protected” mode to ensure RF safety. Additionally, band-specific pTx multiband (MB) pulses with single spokes (corresponding to RF shimming) were designed using the slab-wise design framework 1. To capitalize on the coil geometry and promote RF performance, pulses were designed to image sagittal slices 2. Based on the MB sequence used in the HCP, a new pTx-enabled dMRI sequence was developed to enable application of multiple pTx MB RF pulses for excitation and refocusing, which is an upgrade to our previous sequence only with the ability to apply single pTx MB pulses 3. To demonstrate advantages of pTx over 1Tx, we acquired whole-brain dMRI data with 1.05-mm isotropic resolutions and 2-fold slice acceleration (MB2) as in the 7T HCP dMRI protocol 4, but only considered single-shell q-space sampling and reduced data quantity for simplicity. This dataset consisted of a total of 73 image volumes (including 65 dMRI with b-value=1500 s/mm2 plus 8 interspersed b0 image volumes), each of 144 contiguous sagittal slices. We then sought for optimum slice acceleration for pTx and compared MB2 to higher MB factors of MB3 and MB4. For a more comprehensive comparison, the dMRI data were acquired with double-shell q-space sampling (b-values=1000/2000 s/mm2) as in the 7T HCP dMRI protocol. The same scan time (11 minutes) was spent on data acquisition, resulting in a total of 75, 113 and 135 image volumes for MB2, MB3 and MB4 acquisitions, respectively. All pTx pulse designs were performed in Matlab (Mathworks, USA). In all cases, the q-space sampling schemes were optimized to ensure uniform coverage 5, and the data processing followed the FSL pipeline 6.Results
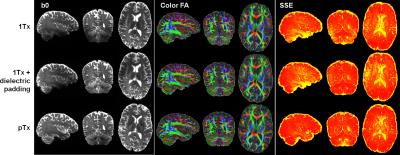
The use of pTx substantially improved RF uniformity across the brain as compared to 1Tx counterparts (Fig. 1). This improvement led to better signal-to-noise ratios especially in inferior brain regions, which in turn translated into better depiction of fiber orientations and less fitting errors across the entire brain (Fig. 2). Additionally, the use of pTx resulted in ~40% less RF power delivery (measured as sum of forward minus reflected power across all channels). The use of dielectric padding for 1Tx acquisition effectively recovered signal in the cerebellum. The use of higher MB factors (only possible with pTx) along with more advanced image reconstruction yielded comparable image quality to MB2 acquisition (Fig. 3). For same scan time, the use of pTx with MB3 to acquire more data than with MB2 yielded better estimation of second fiber orientation with 7% increase in volume and 6% decrease in average dispersion (Fig. 4), and comparable estimation of first fiber orientation (Fig. 5).Discussion
Our results show that pTx can be used to improve the image quality for 7T HCP-type whole-brain dMRI data with ~1-mm isotropic resolutions while reducing RF power deposition or SAR relative to single transmit configurations. The SAR reduction can be traded off for higher slice acceleration, either to increase data for the same amount of scan time or to reduce the scan time for the same amount of data. Our data obtained with higher slice accelerations suggest that, for the Nova 8TxR32 coil, the use of pTx with MB3 may represent the preferred configuration because it outperforms the MB2 acquisition in estimating multiple fiber orientations. The use of MB4 for higher slice acceleration does not provide as good performance; this is in a large part because the TR becomes so short that the signal saturation effect starts to outweigh the gain in data quantity.Conclusion
We have demonstrated the advantages of pTx over single transmit configurations when acquiring HCP-type slice-accelerated high-resolution whole-brain dMRI at 7T. Most notably, pTx can be used to enable higher slice acceleration while improving RF uniformity, thereby enabling acquisition of high quality, high resolution and high efficiency truly whole brain dMRI data that are desired in many neuroscience and clinical applications.Acknowledgements
The authors would like to thank Brian Hanna for setting up computation resources. This work was supported by NIH grants including P41 EB015894 and P30 NS076408.References
1. Wu X, Schmitter S, Auerbach EJ, Ugurbil K, Van de Moortele PF. A generalized slab-wise framework for parallel transmit multiband RF pulse design. Magn Reson Med 2016;75(4):1444-1456.
2. Wu X, Tian J, Schmitter S, Vaughan JT, Ugurbil K, Van de Moortele PF. Distributing coil elements in three dimensions enhances parallel transmission multiband RF performance: A simulation study in the human brain at 7 Tesla. Magn Reson Med 2016;75(6):2464-2472.
3. Wu X, Vu A, Schmitter S, Auerbach E, Moeller S, Lenglet C, Yacoub E, van De Moortele PF, Ugurbil K. Whole brain single shot diffusion weighted EPI at 7 Tesla using parallel transmit multislice multiband RF pulses. Proc Intl Soc Mag Reson Med 22; 2014; Milan, Italy. p 311.
4. Vu AT, Auerbach E, Lenglet C, Moeller S, Sotiropoulos SN, Jbabdi S, Andersson J, Yacoub E, Ugurbil K. High resolution whole brain diffusion imaging at 7T for the Human Connectome Project. NeuroImage 2015;122:318-331.
5. Caruyer E, Lenglet C, Sapiro G, Deriche R. Design of multishell sampling schemes with uniform coverage in diffusion MRI. Magn Reson Med 2013;69(6):1534-1540.
6. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. NeuroImage 2012;62(2):782-790.
Figures