0173
Kt-dSTEAM: high resolution diffusion-weighted imaging of the ex vivo human brain using B1+ homogenized STEAM at 9.4T1Cognitive Neuroscience Department, Maastricht University, Maastricht, Netherlands, 2Institut für Neurowissenschaften und Medizin (INM-1), Forschungszentrum Jülich, Jülich, Germany
Synopsis
The investigation of entire human brains post mortem with diffusion MRI is an important research tool. However, the achievable resolutions and contrast are limited by gradient performance, RF-field inhomogeneity and strongly reduced T2 and diffusivity. Here, a diffusion-weighted STEAM sequence was modified to enable the use of kT-points B1+ homogenization and 3D segmented EPI readout. The resulting kT-dSTEAM sequence allows for high resolution (1000μm, 500μm and 400μm isotropic) diffusion-weighted imaging the entire human brain with homogenous contrast at 9.4T.
Introduction
Ex vivo diffusion MRI (dMRI) is an important research tool in the human brain for neuroanatomical investigations1-3 and the validation of in vivo diffusion MRI techniques. For instance ex vivo dMRI studies of the human brain have focussed on validation of white matter orientation estimates, the atlasing and mapping of large subcortical structures and the delineation of layered grey matter structure4-5. All of these applications benefit from mesoscale (< 1mm isotropic) dMRI resolution over large fields of view, which is difficult to achieve primarily because of the the strongly reduced T2 and diffusivity of fixed tissue. In addition, the investigation of entire human brains post mortem in ultra-high field (UHF) large bore systems6-8, in contrast to small samples studied in small-bore preclinical systems, can provide the crucial context of the complete human brain. However, the achievable resolutions and contrast are limited, compared to small sample studies, by gradient performance, non-optimized RF-coils, and RF-field inhomogeneity over the brain at UHF. Here we introduce kT-dSTEAM with the aim of tackling these challenges and acquiring high resolution (1000μm, 500μm and 0.4μm isotropic) diffusion-weighted images of the entire human brain post-mortem.Methods
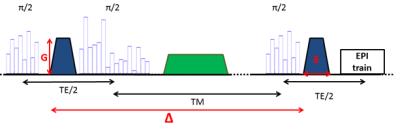
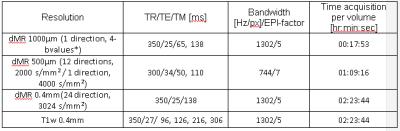
A diffusion-weighted STEAM sequence10 was modified to enable the use of kT-points, leading to the sequence proposed here: kT-dSTEAM. It consists of a set of globally non-selective composite pulses for excitation (with 8 sub-pulses), storing (with 16 sub-pulses) and recalling (with 8 sub-pulses) magnetization for B1+ homogeneity, and a 3D segmented EPI readout acquisition (Figure 1). For the kT-points pulse design a B0 map11 and transmit profile (B1+) map for each of the transmit channels were acquired with transmit phase-encoded12, T2 and T2* compensated version of DREAM13. kT-points sub-pulse spacing was set to 180μs and pulses were calculated using the MLS approach14. For reference, 400μm rectangular pulses with the transmit coil's default static phase setting was used. The kT-dSTEAM sequence takes advantage (compared to pulsed gradient spin echo (PGSE) sequences) of the fact that the mixing time (TM) increases diffusion time (Δ), and hence the b-value, during T1-decay while allowing to keep the echo time (TE) short15. One human post-mortem brainfrom a subject without neurological or psychiatric disease was used for the present study. The sample was enclosed in a 3D conformal container and inserted into a specialized 9.4T 8Ch parallel transmit (pTx), 24Ch receive RF-coil9. Experiments were performed on a 9.4T 82cm bore human MR system (Magnetom 9.4T, Siemens Healthcare, Erlangen, Germany) with a maximum gradient amplitude of 80mT/m and 16 transmit channels. High resolution diffusion-weighted images (at 1000μm, 500μm and 400µm isotropic) were acquired using the parameters specified in table 1. Diffusion tensor imaging (DTI) analysis was performed using FSL v5.0 software16.Results and discussion
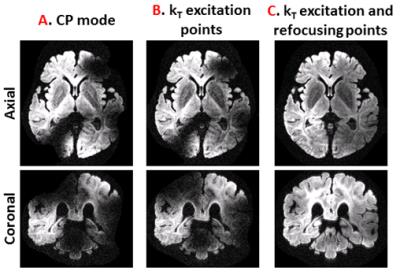
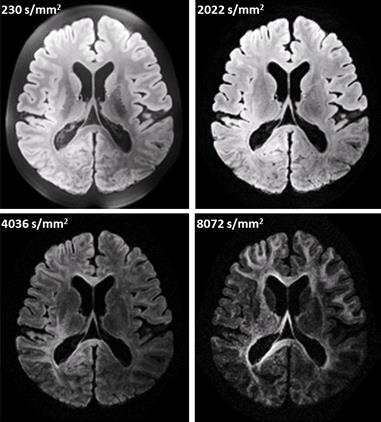
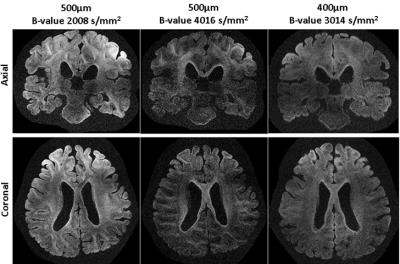
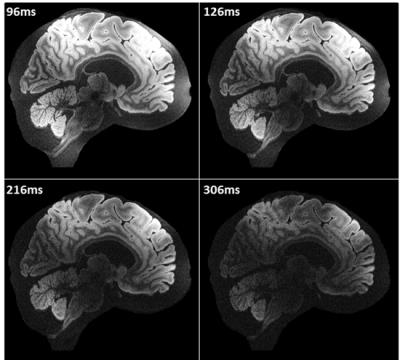
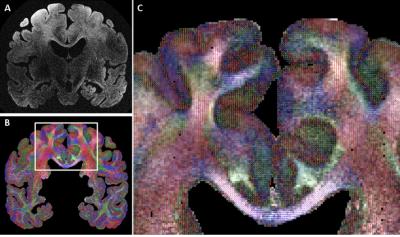
Figure 2A shows that whole brain diffusion-weighted data at 9.4T is highly affected by B1+ inhomogeneity using the static phase RF pulse. Using kT-points excitation alone is not enough to prevent dropout in the stimulated echo signal (Figure 2B), which instead requires the use of composite pulses int storing and recalling (Figure 2C)17. At high resolution (1000µm isotropic) diffusion contrast-to-noise is appreciable up to high b-values of 8072 s/mm2 (Figure 3). Very high resolution diffusion data (500μm and 400μm isotropic) could be achieved at moderate b-values (Figure 4), where adjustment of sequence parameters (TR, TE, TM, RO-bandwidth and EPI factor) allows a trade-off between spatial resolution and diffusion weighting contrast. T1 relaxometry could be also be performed at 400μm by varying TM (Figure 5). The 400µm isotropic data provides DTI orientation estimates in white matter and across the depth of the cortex showing clear radial diffusion (Figure 6).Conclusion
The kT-dSTEAM sequence allows for high resolution diffusion-weighted imaging the entire human brain with homogenous contrast at 9.4T due to the B1+ homogeneity achieved with the kT-points pulses. T1 relaxometry could also be performed a high resolution, showing potential for quantitative T1 mapping. Acquisition time could be shortened in the future by application of accelerated sampling schemes in the EPI readout. With these further developments kT-dSTEAM can play an important role in mesoscale human connectomics and microstructure studies.Acknowledgements
The authors thank Katrin Amunts for making available the human brain specimen.References
1. Conturo, T. E., Lori, N. F., Cull, T. S., et al. Tracking neuronal fiber pathways in the living human brain. Proc. Natl. Acad. Sci. USA 1999; 96, 10422–10427.
2. Dell'Acqua F, et al. MR diffusion histology and micro-tractography reveal mesoscale features of the human cerebellum. Cerebellum, 2013; 12(6): 923-931.
3. Aggarwal M, et al. Probing region-specific microstructure of human cortical areas using high angular and spatial resolution diffusion MRI. Neuroimage, 2015; 105:198-207.
4. Leuze C.W, et al. Layer-specific intracortical connectivity revealed with diffusion MRI. Cereb Cortex, 2014; 24(2): 328-339.
5. Roebroeck A, et al. High-resolution diffusion tensor imaging and tractography of the human optic chiasm at 9.4 T. Neuroimage, 2008; 39(1): 157-168.
6. Foxley S, et al. Improving diffusion-weighted imaging of post-mortem human brains: SSFP at 7T. Neuroimage, 2014; 102 (2): 579-589.
7. Miller K.L, et al. Diffusion imaging of whole, post-mortem human brains on a clinical MRI scanner. Neuroimage, 2011; 57(1): 167-81.
8. Yang, S, et al. Integration of ultra-high field MRI and histology for connectome based research of brain disorders. Front Neuroanat, 2013; 7: 31.
9. Roebroeck A. et al. High resolution MRI neuroanatomy of the whole human brain post mortem with a specialized 9.4T RF-coil. Proc. OHBM 2015 Poster #1856
10. Merboldt KD, Hanicke W, Frahm J. Diffusion imaging using stimulated echoes. Magn. Reson. in Med. Jun 1991;19(2):233-239.
11. Heiland S., Dietrich O., Sartor K. Diffusion-weighted imaging of the brain: comparison of the stimulated- and spin-echo-planar sequence. Neuroradiology 2001; 43: 442-447
12. Tse DH, Wiggins CJ, Ivanov D, et al. Volumetric imaging with homogenised excitation and static field at 9.4 T. Magn Reson Mater Phy 2016; 29 (3):333-345
13. Tse DHY, Poole MS, Magill AW, et al. Encoding methods for B1(+) mapping in parallel transmit systems at ultra high field. J Magn Reson 2014; 245:125-132
14. Nehrke K. and Bornert P. DREAM--a novel approach for robust, ultrafast, multislice B(1) mapping. Magn Reson Med, 2012; 68(5): 1517-1526.
15. Setsompop K, et al. Magnitude least squares optimization for parallel radio frequency excitation design demonstrated at 7 Tesla with eight channels. Magn Reson Med, 2008; 59(4): 908-915.
16. Jenkinson M, et al. FSL. NeuroImage, 2012; 62:782-90.
17. Eggenschwiler F., et al. Improving T2-weighted imaging at High Field through the use of kT-points. Magn Reson Med, 2014; 71: 1478-1488
Figures