0170
Measurement of metabolic changes in acute doxorubicin-induced cardiotoxicity in mice using hyperpolarized [1-13C]pyruvate1Division of Cardiology, University Hospital Lausanne (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 2Institute of Physics, Swiss Federal Institute of Technology (EPFL), Lausanne, Switzerland, 3Department of Radiology, Hospital Lausanne (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland
Synopsis
Chemotherapy cocktails containing doxorubicin produce irreversible cardiotoxic side effects that may progress to heart failure, which can only be avoided through dose limitation of the chemotherapeutic agents. Increasing evidence suggest that cardiac dysfunction caused by doxorubicin is triggered by an energetic deficit and alterations in mitochondrial metabolism. We quantified metabolic changes in vivo in a mouse model of acute doxorubicin-induced cardiotoxicity using hyperpolarized 13C MRS.
Purpose
Chemotherapy cocktails containing anthracyclines such as doxorubicin (DOX) often lead to significant irreversible side effects that can lead to heart failure [1,2]. DOX is widely used as a chemotherapy agent and its cardiotoxic side effects are detrimental for cancer treatment as they can only be avoided through dose limitation. Increasing evidence suggest that the myocardial injury caused by DOX is preceded by metabolic changes in the heart originating from mitochondrial dysfunction. There is thus a need to understand the metabolic alterations that relate to cardiotoxic processes in vivo to improve treatment outcomes by decreasing the cardiovascular risk. The quantification of metabolic changes is challenging in vivo, however the development of hyperpolarized (HP) 13C magnetic resonance spectroscopy (MRS) has made such measurements possible [3] and the first clinical cardiac studies have just recently been reported [4]. The aim of this study was to determine the presence of metabolic changes during acute doxorubicin induced cardiotoxicity in mice, and whether HP 13C MRS is sufficiently sensitive for the detection of such changes in vivo.Methods
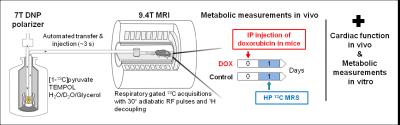
Male C57Bl6/J mice (n=10) were divided in two groups, a control group (Control) that received intraperitoneal (IP) injections of a saline solution and a group receiving a single injection of DOX at 15 mg/kg . Hyperpolarized 13C MRS was performed one day after treatment. A 100 µL sodium [1-13C]pyruvate (2.6 M) solution containing H2O/D2O/glycerol (3:3:2 ratio) and TEMPOL free radical (80 mM) was frozen into beads and dynamically hyperpolarized in a custom-built 7 T polarizer at 1 K with 197.14 GHz for 1.5 hr. The hyperpolarized (HP) sample was dissolved in 6 mL of preheated buffer and transferred to a separator/infusion pump located inside the MRI scanner (9.4T) using a fully-automated procedure. A total volume of 350 µL hyperpolarized solution was then infused in 2 s into the femoral vein of the mouse via a microcatheter with a dead volume of 125 µL. Respiratory-gated 13C spectra were acquired using adiabatic 30° pulses applied with a repetition time of ~3 seconds and 1H decoupling using a single loop 13C coil combined with a quadrature 1H coil. Acquired spectra were analyzed by fitting in Bayes (Washingtom University, St. Louis) to determine metabolite ratios.
A second cohort of male C57Bl6/J mice subjected to the same protocol was used in parallel to determine changes in cardiac function using echocardiography, associated with changes in mitochondrial activity that were assessed with high resolution respirometry in whole heart homogenates.
Statistics were computed via unpaired two-tailed Student’s t-tests with equal variance.
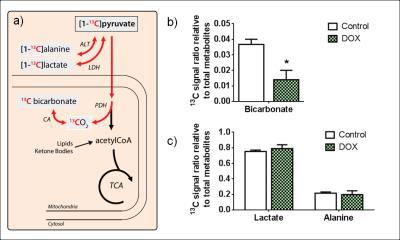
Results and Discussion
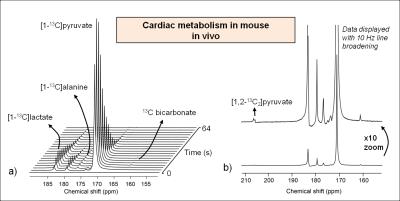
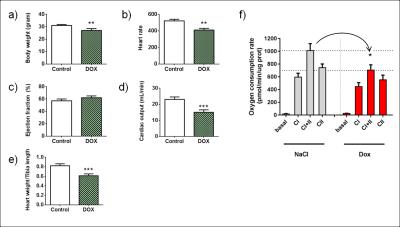
Following the metabolism of hyperpolarized [1-13C]pyruvate, 13C label incorporation was rapidly detected in the downstream metabolites lactate, alanine and bicarbonate (Fig. 2a). HP 13C metabolites were on average visible for a time period of 66 ± 13 s. The spectral resolution obtained at 9.4T combined with the narrow linewidths of metabolites allows clear separation of different resonances (Fig. 2b). The decarboxylation of pyruvate through pyruvate dehydrogenase (PDH) produced 13C labeled CO2 that equilibrates with 13C bicarbonate (Fig. 3a), which provides a measure of carbohydrate metabolism in general and their relative contribution to the tricarboxylic acid (TCA) cycle. Analysis of the metabolite ratios computed from summed spectra revealed significant (p<0.05) changes in 13C bicarbonate labeling that indicates a decrease in PDH flux. No changes in 13C labeling in alanine and lactate were observed in this preliminary study, although changes in alanine have been observed after a four-week DOX treatment period in rats [5]. DOX-treatment caused, 2 days post injection, a decrease in body weight (Fig. 4a), and a decrease in heart rate and cardiac output which are indicative of cardiac systolic dysfunction (Fig. 4b-d). Cardiotoxicity was also associated with cardiac atrophy as evidenced by the decreased heart weight/tibia length ratio (Fig. 4e). A decrease of 30% in the oxygen consumption rate was observed in cardiac tissues from DOX-treated animals (Fig. 4f). To summarize, these cardiac function measurements indicate a myocardial injury caused by the administration of DOX.Conclusion
Hyperpolarized 13C MRS can be used to detect and quantify, in vivo, early metabolic changes in mice that are caused by acute doxorubicin-induced cardiotoxicity. The decreased PDH flux reflects a myocardial injury as a result of doxorubicin administration and is also correlated with measurements of decreased mitochondrial activity. Restoring the PDH flux in doxorubicin-induced cardiotoxicity might be a promising target for pharmacological intervention to avert cardiac dysfunction.Acknowledgements
The authors would like to thank the CIBM, Nanotera, and the Emma Muschamp FoundationReferences
[1] Lotrionte et al., Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. The American journal of cardiology 112(12):1980-1984 (2013)
[2] Tokarska-Schlattner et al., New insights into doxorubicin-induced cardiotoxicity: the critical role of cellular energetics. Journal of molecular and cellular cardiology 41(3):389-405 (2006).
[3] Ardenkjaer-Larsen et al., Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proceedings of the National Academy of Sciences of the United States of America 100(18):10158-10163 (2003)
[4] Cunningham et al., Hyperpolarized 13C Metabolic MRI of the Human Heart: Initial Experience. Circulation Research (2016) http://dx.doi.org/10.1161/CIRCRESAHA.116.309769
[5] Dodd et al., Proc. Intl. Soc. Mag. Reson. Med. 24, 2536 (2016)
Figures