0168
Hyperpolarized 13C Magnetic Resonance Evaluation of Renal Ischemia Reperfusion Injury in a Murine Model1Department of Radiology, Leiden University Medical Center, C.J. Gorter Center for High-field MRI, Leiden, Netherlands, 2Department of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 3Department of Surgery, University of California San Francisco, San Francisco, CA, United States, 4Department of Medicine, University of California San Francisco, San Francisco, CA, United States
Synopsis
Persistent oxidative stress and mitochondrial dysfunction have been implicated across diverse forms of acute kidney injury and in the transition to chronic kidney disease. We show that HP 13C metabolic MR can be used to noninvasively assess the altered renal redox capacity and mitochondrial PDH activity following ischemic reperfusion injury. Such an imaging approach can potentially enhance the prediction and monitoring of progressive kidney injury.
BACKGROUND
Acute kidney injury (AKI) is a major complication in hospitalized patients, and often leads to chronic kidney disease (CKD). Persistent oxidative stress and mitochondrial dysfunction have been implicated across diverse forms of AKI and in the transition to CKD. Biomarkers that can noninvasively inform on the above may improve the risk stratification of patients following AKI and aid in the assessment of targeted therapy efficacy. Hyperpolarized (HP)-13C-MR spectroscopy and imaging have enabled noninvasive visualization of normal and abnormal metabolism in living systems. In this study, we applied HP-[1-13C]-dehydroascorbate (DHA) and [1-13C]-pyruvate MR to investigate the renal redox capacity and mitochondrial PDH activity, respectively, in a murine model of AKI induced by unilateral ischemic/reperfusion injury (IRI).METHODS
FVB/N mice (n=15, 3-6 mo) were scanned at baseline and 7 days after being subjected to a 40 min unilateral IRI. HP-[1-13C]-DHA MRS was acquired on a 3T GE Healthcare system using a 1H-13C quadrature coil. HP-[1-13C]-DHA was prepared as previously described,1 and polarized on a HyperSense (Oxford Instruments). The dissolved compound (250μL, 21mM) was injected over 15s through a tail vein catheter. 3D 13C CSI was acquired 25s after the beginning of the injection (3D spin-echo, ramp-sampled symmetric EPSI readout with variable flip angle schedule, 6x6x6mm3 resolution). Data were reconstructed in matlab and visualized in sivic. 2 Results are expressed as peak height VitC/(VitC+DHA) ratio. HP 13C-pyruvate MRS was performed on a vertical 14.1T Varian system with a 1H-13C quadrature coil (M2M). HP 13C-pyruvate (350μL, 160mM) was injected over 10s through the tail vein. 2D-CSI was acquired 25s after the beginning of the injection (slice thickness 8mm, matrix size 8x8, FOV: 30x30 mm2, acquisition time 8s) with a 350MHz frequency offset from pyruvate. FIDs were processed in MestReNova. After phase and baseline correction, lactate (Lac), alanine (Ala), pyruvate (Pyr) and bicarbonate (Bic) peaks were integrated. Arterial spin labeling 1H MR was performed at 14.1T to measure renal perfusion using pre-saturated pulsed ASL-FSE3 with fat suppression and respiration triggering3,4 (inversion time: 1.5s, echo-train length: 32, inter-TE: 2.8ms; FOV: 30x30mm2; matrix size: 128x128, slice thickness: 2mm, 30 averages). The slice of interest was 9mm off-centered to ensure RF coverage of the heart. Perfusion maps were generated in Matlab. Reactive oxygen species (ROS) staining was performed on fresh renal cortical frozen sections (8 µ) with a fluorescent dye, 2’-7’-DCF-diacetate (DCF) (4µM, Invitrogen/Molecular Probes) and confocal microscopy. Renal tubular injury was assessed using Periodic Acid Schiff (PAS) staining. Pyruvate dehydrogenase (PDH) activity, lactate dehydrogenase (LDH) activity and serum blood urea nitrogen (BUN) were measured using commercially available kits (Abcam and Arbor Assays).RESULTS and DISCUSSION
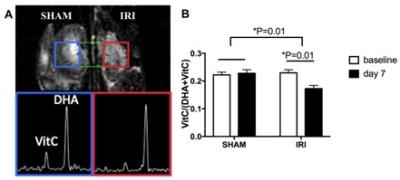
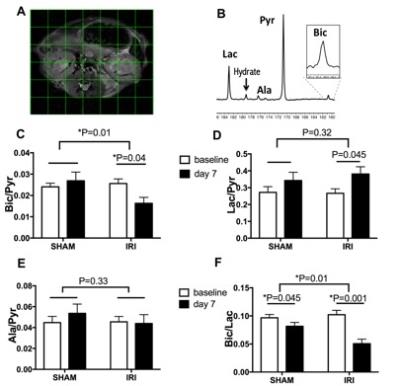
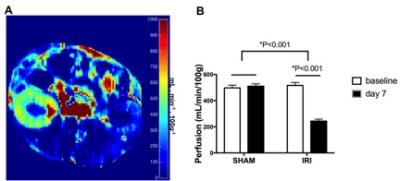
The kidneys subjected to IRI showed significantly lower VitC/(VitC+DHA) ratio on day 7 compared to baseline (p=0.01) and compared to SHAM (p=0.01), consistent with lower redox capacity (Figure 1). The 13C-Bic/Pyr ratio decreased significantly from baseline to day 7 in IRI (p=0.04) consistent with impaired PDH activity, and the change in the Bic/Pyr ratio was significantly different between IRI and SHAM (p=0.01). The 13C-Lac/Pyr ratio increased significantly from baseline to day 7 in IRI (p=0.045). However, the difference in the 13C-Lac/Pyr ratio between IRI and SHAM was not significant (p=0.32). The 13C-Ala/Pyr ratio remained the same following IRI. We also evaluated the ratio of 13C-Bic/Lac as another measure of oxidative pyruvate metabolism that is independent of the 13C-pyruvate delivery. The 13C-Bic/Lac ratio decreased significantly from baseline to day 7 in both IRI and SHAM (p=0.001 and 0.045 respectively), and the difference in the 13C-Bic/Lac ratio was significant between IRI and SHAM (p=0.01). Seven days after unilateral IRI, perfusion was significantly decreased in the injured kidneys (p<0.001) and remained largely unchanged in the contralateral kidney. BUN levels were significantly higher at day 7 compared to baseline, indicating renal tubular dysfunction (p<0.005). The IRI kidneys had significantly higher injury score on histology compared to the contralateral kidneys (2.4±0.8 vs. 0.5±0.5, p<0.001). DCF showed markedly stronger ROS staining in IRI compared to SHAM, consistent with our HP-[1-13C]-DHA data. PDH activity was significantly lower in IRI than in SHAM at day 7 (30.5±29.6 vs 154.7±32.2 ∆mOD450/mg protein per min, p=0.005), corresponding to the HP pyruvate data. There were no significant differences in the LDH activity (Km, Vmax) between IRI and SHAM at day 7 (p=0.45 and 0.19 respectively).CONCLUSION
We have shown that HP 13C metabolic MR can be used to assess noninvasively the altered renal redox capacity and mitochondrial PDH activity following IRI. Such an imaging approach can potentially enhance the prediction and monitoring of progressive injury, as well as providing companion biomarkers of targeted therapies.Acknowledgements
CB and HQ contributed equally. This work was funded by American Heart Association and Department of Veterans Affairs, NIH NIDDK RO1 DK097357, and NIH P41EB013598References
1- Keshari KR, Kurhanewicz J, Bok R, Larson PEZ, Vigneron DB, Wilson DM. Hyperpolarized 13C dehydroascorbate as an endogenous redox sensor for in vivo metabolic imaging. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(46):18606-18611. doi:10.1073/pnas.1106920108.
2- Crane JC, Olson MP, Nelson SJ. SIVIC: Open-Source, Standards-Based Software for DICOM MR Spectroscopy Workflows. International Journal of Biomedical Imaging. 2013;2013:169526. doi:10.1155/2013/169526.
3- Pell, G. S., Thomas, D. L., Lythgoe, M. F., Calamante, F., Howseman, A. M., Gadian, D. G. and Ordidge, R. J. (1999), Implementation of quantitative FAIR perfusion imaging with a short repetition time in time-course studies. Magn. Reson. Med., 41: 829–840. doi:10.1002/(SICI)1522-2594(199904)41:4<829::AID-MRM24>3.0.CO;2-U
4- Duhamel, G., Prevost, V., Girard, O. M., Callot, V. and Cozzone, P. J. (2014), High-resolution mouse kidney perfusion imaging by pseudo-continuous arterial spin labeling at 11.75T. Magn. Reson. Med., 71: 1186–1196. doi:10.1002/mrm.24740
Figures