0166
Effects of 3-MPA on in vivo hepatic metabolism of hyperpolarized [1-13C] pyruvate1Institute of Physics, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2Division of Cardiology, University Hospital Lausanne (CHUV), Lausanne, Switzerland, 3Laboratory for Functional and Metabolic Imaging, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 4Department of Radiology, University of Lausanne (UNIL), Lausanne, Switzerland
Synopsis
Ex vivo and in vivo studies on liver metabolism using hyperpolarized [1-13C]pyruvate report do not agree on whether hyperpolarized bicarbonate metabolite production results from pyruvate oxidation or gluconeogenesis. This study tested the ability of hyperpolarized [1-13C]pyruvate to probe gluconeogenesis in the liver of intact rats. While conversion to hyperpolarized bicarbonate was detected in the liver of fasted rats, treatment with the phosphoenolpyruvate carboxykinase inhibitor 3-mercaptopicolinc acid resulted in 7-fold lower levels. This result supports the notion that hepatic gluconeogenic metabolism can indeed be directly probed in vivo with hyperpolarized pyruvate.
Introduction
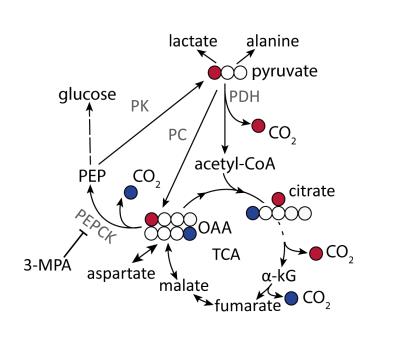
Hyperpolarized (HP) [1-13C]pyruvate is a useful probe to investigate liver bio-energetic metabolism1-3. However, it has been challenging to distinguish its relative contributions to gluconeogenesis (GNG) and to the citric acid cycle via pyruvate oxidation since both pathways result in the conversion of [1-13C]pyruvate to 13C-bicarbonate4. While oxidative decarboxylation by pyruvate dehydrogenase (PDH) directly produces 13CO2 from [1-13C]pyruvate, its formation via gluconeogenesis requires at least six enzymatic steps, culminating in the decarboxylation of [4-13C]oxaloacetate by phosphoenolpyruvate carboxykinase (PEPCK) (Fig. 1). This convoluted pathway and the fact that relatively little labeled bicarbonate is produced under fasting conditions, when the relative flux of pyruvate through gluconeogenesis is higher, has led to the conclusion that the HP 13C-bicarbonate detected in the liver in vivo results from PDH flux5. By contrast, the conversion of pyruvate label to bicarbonate in the fatty-acid perfused liver of mice is reported to result from PEPCK activity2,4. This study aimed to test the hypothesis that conversion of HP [1-13C]pyruvate to bicarbonate observed in vivo in the fasted rat liver results from gluconeogenic flux, using the PEPCK inhibitor 3-mercaptopicolinic acid (3-MPA)6 to modulate this pathway.Materials and Methods
Polarization: Neat [1-13C]pyruvic acid doped with OX063 trityl radical (Albeda, Denmark) was polarized in a 7 T custom-built DNP polarizer at 1.00 ± 0.05 K and 196.8 GHz using a nominal output microwave power of 55 mW. After 1.5 hr, the frozen sample was rapidly dissolved with 6 ml preheated deuterated phosphate buffer (~pH 7.5). The HP solution was automatically transferred into a separator/infusion pump located inside the animal bore.
Animals: Male Sprague Dawley rats (~200g, n=3 for each group) were anaesthetized with isoflurane (1-2%). Catheters for substrate administration and invasive blood pressure measurements were installed in a femoral vein and artery, respectively. Arterial blood samples were taken to determine glucose and plasma insulin levels 10 min before the injection of HP [1-13C]pyruvate.
PEPCK inhibition: A 0.5 ml solution of 3-MPA (Toronto Research Chemicals, Canada) at neutral pH was administered by intraperitoneal (IP) injection (100 mg 3-MPA/kg body weight) over 1 min, 1 hr prior to the HP [1-13C]pyruvate infusion.
MR acquisition: A 1 mL bolus of 0.066± 0.007 mmol/kg HP [1-13C]pyruvate was administered intravenously in 9 s to fed and overnight fasted rats. In vivo 13C MRS measurements were respiration and cardiac gated with a repetition time of ~3 s in a 9.4T/31cm horizontal bore magnet with a VNMRS console (Varian/Magnex, USA). 30° BIR4 adiabatic RF excitation pulses were applied with a custom-built quadrature 1H/single loop 13C surface coil placed over the liver of the rat and 13C FIDs acquired with 1H decoupling.
Data analysis: The metabolite peak integrals were quantified and normalized with the total 13C signal of the metabolites analyzed using VNMRJ (Agilent, USA). Error bars indicate ± SEM. Metabolite ratios were compared between 3-MPA-treated and non-treated (control group) rats in each nutritional condition independently and analyzed using a Student's t-test. Differences between groups were considered significant if p<0.05.
Results and Discussion
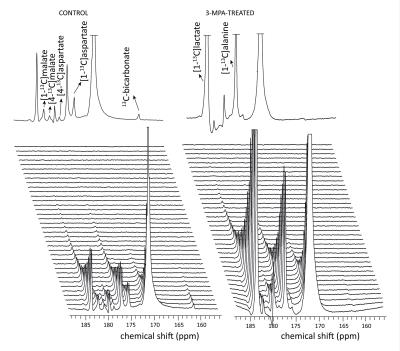
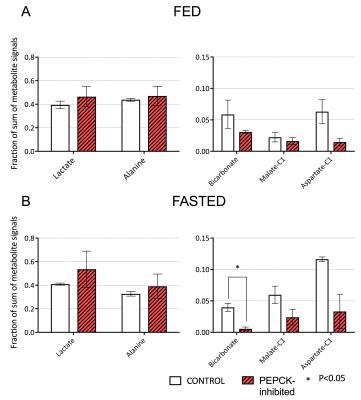
Conversion of infused HP [1-13C]pyruvate to 13C-bicarbonate, [1-13C]alanine, [1-13C]lactate, [1-13C]aspartate, and [1-13C]malate was detected in all animals (Fig 2). While lactate and alanine remained roughly unchanged, aspartate and malate were both lower in the 3-MPA-treated group, reflecting a lower metabolite pool size and/or diminished pyruvate carboxylate flux (Fig 3). [4-13C]malate and [4-13C]aspartate were not consistently detectable in the treated group. In fed rats, bicarbonate was almost 2-fold lower with 3-MPA treatment, while it was 7-fold lower in the fasted group. This substantial decrease in 13C-bicarbonate levels in fasted animals (Fig 3) is consistent with bicarbonate production resulting from PEPCK activity, which predominates over pyruvate oxidation in this nutritional state. The residual bicarbonate in 3-MPA-treated fasted-rat liver may result from PDH activity or incomplete PEPCK inhibition, while the insignificant decrease in the fed state may reflect PEPCK inhibition and/or diminished PDH flux.Conclusion
We investigated the effects of GNG inhibition via blockage of PEPCK on changes in pyruvate utilization in different nutritional conditions. Pyruvate carboxylate predominates in fasted liver as HP bicarbonate levels significantly decreased in PEPCK inhibited groups. Further studies are needed in fed animals to investigate the PEPCK inhibition and GNG.Acknowledgements
This work was supported by the Swiss National Science Foundation (PP00P2_133562), the FP7 Marie Curie Initial Training Network (ITN) METAFLUX and by the Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL, and the Leenards and Jeantet Foundations.References
[1] Hu S. et al. Mol. Imag. Biol 11:399-407 (2009)
[2] Merritt M. et al. PNAS 47: 19084–19089 (2011)
[3] von Morze C. et al. Magnetic resonance in medicine (2016)
[4] Moreno K. X. et al. Metabolomics, 11(5), pp.1144-1156 (2015)
[5] Jin E. S. et al. NMR Biomed. 29(4):466–74. (2016)
[6] DiTullio N.W. et al. Biochemical Journal, 138(3), pp.387-394 (1974)
Figures