0163
Long-lasting, liquid-state 13C hyperpolarization > 20 % generated in an MRI system within seconds enables fast 13C imaging1University Medical Center Freiburg, Freiburg, Germany, 2Fraunhofer Institute for Solar Energy Systems (ISE), Freiburg, Germany, 3German Consortium for Cancer Research (DKTK), Heidelberg, Germany
Synopsis
Current methods for the production of hyperpolarized 13C-tracers require a dedicated, complex and costly polarizer device. Here we present, for the first time, 13C-hyperpolarization >20% and ex-vivo 13C-MRI without an external polarizer, but by using the hardware of an MRI system instead: a simple, low-cost (≈1000€) setup was built and high-field spin-order-transfer sequences were exploited to transfer the spin-order of parahydrogen to 13C;the implementation on any multinuclear MRI system appears feasible. The tracer is produced near the application site and subsequent 13C-MRI is possible without transfer of the sample, at a fraction of the cost and complexity of external polarizers.
Introduction
Nuclear hyperpolarization (HP) has increased the sensitivity of MRI to monitor metabolism non-invasively and in vivo.1–3 To date, HP-tracers are produced in an external, complex and costly polarizer device. Moreover, some of the enhancement of the short-lived HP-tracer is lost during the (field-)transfer to the application site. Here we demonstrate that 13C-HP of (21±2)% can be produced within seconds inside an MRI system, next to the application site by means of parahydrogen (pH2).4 No external polarizer is needed and transfer of the tracer is no longer required. HP at the application site may allow for a higher polarization at the time of detection, at a fraction of the cost and complexity of external polarizers.Methods
pH2 was enriched to ≈95% as described previously.5
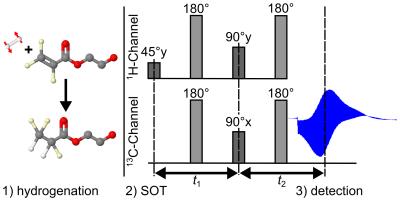
Samples were prepared similarly to previous reports using 13C-labeled hydroxylethyl acrylate (HEA) as precursor molecule.6,7 During HP hydroxylethyl propionate (HEP) was formed after para-hydrogenation.
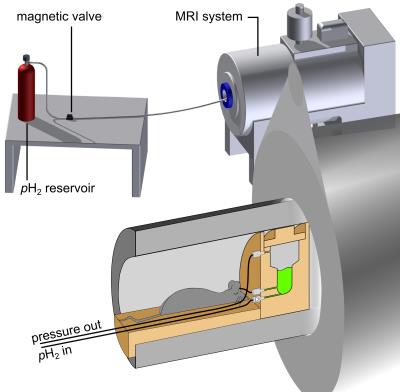
Setup: A 7T preclinical MRI system (Biospec 7/20, PV5.1, Bruker, Germany) and a dual-resonant 1H-13C transmit-receive volume coil (Rapid, Germany) were used for HP and MRI. A high pressure and high temperature reactor was custom-made to yield fast hydrogenation (inner volume ≈2ml, PSU 1000)(Fig.1).
Spin order transfer (SOT): PH-INEPT+8 and l-PH-INEPT+9 sequences were implemented on the MRI system to transfer HP from 1H to 13C. Note that only l-PH-INEPT+ enabled production of an HP-tracer as longitudinal magnetization decays much slower (with T1 instead of T2).
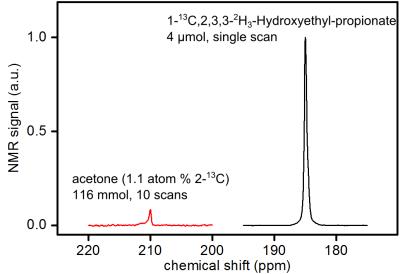
Hyperpolarization: The heated reactor (≈80°C) was filled with the hot precursor solution (≈80°C), closed and placed in the magnet. Hydrogenation was initialized by injecting pressurized pH2 (≈15-30bar) from the bottom of the reactor. After a variable hydrogenation time th the SOT was executed (Fig.2). 13C-HP was quantified assuming 100% hydrogenation and 100% pH2-enrichment, using an 8ml sample of thermal acetone.
Ex vivo experiments: To demonstrate simultaneous HP and MRI, an ex-vivo rat (rattus rattus, 35g,7cm,7 days old) was connected to the reactor using a catheter and placed next to the chamber. About 700µl of HP-tracer were produced and subsequently, ≈330µl of it were injected into the thorax of the rodent.
Results
The highest 13C-polarization after the PH‑INEPT+ sequence was quantified to P≈24%, corresponding to an enhancement factor of η≈40,000 at 7T (Fig.3,HEP concentration cHEP=5.54mM,p=15bar,th=4s,catalyst concentration ccat=2.1mM,H2O). The inter-day reproducibility was quantified to PHP≈(21±2)%(N=9). When the concentration of the agent was increased, polarizations of P≈17% and P≈13% were achieved for 22mM (p=15bar,th=8s,ccat=4.2mM,H2O) and 80mM (p=30bar,th=8s,ccat=4.2mM,H2O), respectively.
As predicted by Bär et al.,9 the l-PH-INEPT+ sequence enabled the production of longitudinal HP-magnetization of a similar order (P=19±2% 2s after SOT,cHEP=5.54mM,15bar,th=4s,ccat=2.1mM,D2O). The T1 of HEP at 7T was measured to (79±2)s and (75±5)s, when dissolved in D2O or H2O, respectively.
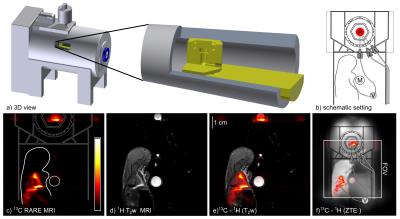
13C-MRI of an ex-vivo rat was performed within seconds, without leaving the magnet and strong 13C-HP signal was observed (P≈17%,cHEP=22mM,p=15bar,th=8s,ccat=4.2mM,H2O; single-shot RARE,90/180°,RARE-factor:38,FOV:(8.4cm)2,zerofilled to 1282 matrix, in-plane resolution:(0.65 mm)2, slice thickness:6cm,TR=0.487s,TE=79ms,acquisition time:0.487s). 1H-MRI showed that the 13C signal was localized around the heart of the rat and within the reactor, yielding a maximum 13C-SNR of 113 and 111, respectively (T2-weighted 1H-Turbo-RARE,90/180°,RARE-factor:8,matrix:2562,in-plane resolution:(328mm)2,TR=2.5s,TE=33ms, acquisition time:80s). In the same scan, the SNR of a model solution was quantified to 9 (333mg 1-13C sodium-acetate,1.2ml deionized H2O,c=3.3M). A ZTE-MRI was acquired afterwards to depict the entire setup (2.3°,matrix:1283,FOV:16.8cm,TR=4ms,acquisition time:208s, projections:51896)(Fig.4).
Discussion
Highly polarized and concentrated 13C-labeled tracers were produced within seconds, in aqueous solution and within the magnet bore near the application site. For the first time, high 13C-HP was achieved without a dedicated polarizer, but by using the hardware of an MRI system instead.
Whereas the observed HP-levels are sufficient for biomedical use,1,10 they may be further increased by optimization of, e.g., the parameters of the hydrogenation reaction (p, ccat and temperature). Although, some challenges persist for all pH2-based HP-methods, i.e. obtaining a pure solution devoid of catalyst and a high HP of biologically relevant tracers, great advances have been made regarding these former drawbacks in recent years.11–18
Our method circumvents some of the impediments of current 13C-HP methods, in particular the need of a dedicated, expensive and complex external polarizer and lengthy transfer of the tracer.
Conclusion
Considering the fast production and high polarization yield, the recently extended portfolio of pH2-tracers, including pyruvate and acetate, phospholactate and succinate, the low cost (≈103€), simplistic design and operation, our method may substantially facilitate the research on and application of HP tracers.Acknowledgements
Funding support by the DFG (HO 4604/1-1 and HO 4604/2-1), the German Consortium for Translational Cancer Research and the contribution of the COST Action TD1103 are gratefully acknowledged.References
1. Ardenkjaer-Larsen, J. H. et al. Dynamic nuclear polarization polarizer for sterile use intent. NMR Biomed. 24, 927–932 (2011).
2. Nelson, S. J. et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C]Pyruvate. Sci. Transl. Med. 5, 198ra108 (2013).
3. Cunningham, C. H. et al. Hyperpolarized 13C Metabolic MRI of the Human Heart: Initial Experience. Circ. Res. CIRCRESAHA.116.309769 (2016).
4. Bowers, C. R. & Weitekamp, D. P. Parahydrogen and synthesis allow dramatically enhanced nuclear alignment. J. Am. Chem. Soc. 109, 5541–5542 (1987).
5. Hoevener, J.-B. et al. A continuous-flow, high-throughput, high-pressure parahydrogen converter for hyperpolarization in a clinical setting. NMR Biomed. 26, 124–131 (2013).
6. Hoevener, J.-B. et al. PASADENA hyperpolarization of 13C biomolecules: equipment design and installation. Magn Reson Mater Phy 22, 111–121 (2009).
7. Hoevener, J.-B. et al. Quality assurance of PASADENA hyperpolarization for 13C biomolecules. Magn Reson Mater Phy 22, 123–134 (2009).
8. Haake, M., Natterer, J. & Bargon, J. Efficient NMR Pulse Sequences to Transfer the Parahydrogen-Induced Polarization to Hetero Nuclei. J Am Chem Soc 118, 88–91 (1996).
9. Bär, S. et al. On the spin order transfer from parahydrogen to another nucleus. J. Magn. Reson. 225, 25–35 (2012).
10. Dutta, P., Martinez, G. V. & Gillies, R. J. A New Horizon of DNP technology: Application to In-vivo 13C Magnetic Resonance Spectroscopy and Imaging. Biophys. Rev. 5, 271–281 (2013).
11. Reineri, F. et al. Use of Labile Precursors for the Generation of Hyperpolarized Molecules from Hydrogenation with Parahydrogen and Aqueous-Phase Extraction. Angew. Chem. Int. Ed. 50, 7350–7353 (2011).
12. Glöggler, S. et al. A nanoparticle catalyst for heterogeneous phase para-hydrogen-induced polarization in water. Angew. Chem. Int. Ed Engl. 54, 2452–2456 (2015).
13. Reineri, F., Boi, T. & Aime, S. ParaHydrogen Induced Polarization of 13C carboxylate resonance in acetate and pyruvate. Nat. Commun. 6, 5858 (2015).
14. Chekmenev, E. Y. et al. PASADENA Hyperpolarization of Succinic Acid for MRI and NMR Spectroscopy. J. Am. Chem. Soc. 130, 4212–4213 (2008).
15. Zacharias, N. M., Chan, H. R., Sailasuta, N., Ross, B. D. & Bhattacharya, P. Real-Time Molecular Imaging of Tricarboxylic Acid Cycle Metabolism in Vivo by Hyperpolarized 1-13C Diethyl Succinate. J. Am. Chem. Soc. 134, 934–943 (2012).
16. Shchepin, R. V., Coffey, A. M., Waddell, K. W. & Chekmenev, E. Y. PASADENA Hyperpolarized 13C Phospholactate. J. Am. Chem. Soc. 134, 3957–3960 (2012).
17. Shchepin, R. V., Pham, W. & Chekmenev, E. Y. Dephosphorylation and biodistribution of 1-13C-phospholactate in vivo. J. Label. Compd. Radiopharm. 57, 517–524 (2014).
18. Shchepin, R. V., Coffey, A. M., Waddell, K. W. & Chekmenev, E. Y. Parahydrogen Induced Polarization of 1-13C-Phospholactate-d2 for Biomedical Imaging with >30,000,000-fold NMR Signal Enhancement in Water. Anal. Chem. 86, 5601–5605 (2014).
19. Olsson, L. E. et al. MR coronary angiography in pigs with intraarterial injections of a hyperpolarized 13C substance. Magn. Reson. Med. 55, 731–737 (2006).
Figures