0161
MB-SWIFT functional MRI during deep brain stimulation in rats1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2A. I. Virtanen Institute for Molecular Sciences, University of Eastern Finland, Kuopio, Finland
Synopsis
Commercial electrodes used for Deep Brain Stimulation (DBS) cause severe artefacts in conventional echo based MRI. Here we show near artefact free functional MRI during DBS in rats using Multi-Band SWeep Imaging with Fourier Transformation (MB-SWIFT) which allows acquisition at virtually zero-TE. MB-SWIFT showed strong responses in the somatosensory cortex while stimulating the ventromedial. The amplitude and extent of activation recorded with MB-SWIFT were similar with SE-EPI, although activation was flip angle dependent reflecting the possible influence of blood inflow. MB-SWIFT is a promising modality for fMRI in the presence of DBS leads or other severe susceptibility differences.
Purpose
Being able to detect brain activity during deep brain stimulation (DBS) is essential for understanding the mechanisms underlying the neuromodulation. For this purpose, simultaneous fMRI-DBS studies have been conducted in humans and animals using EPI1,2, however, the electrode can cause significant artefacts and signal loss in EPI pulse sequences due to susceptibility variations. Recently, the 3D radial pulse sequence SWeep Imaging with Fourier Transform (SWIFT) with virtually zero-TE was shown to provide functional contrast in the human brain at 4 T3. In this work, we demonstrate that fMRI maps nearly free of susceptibility artifacts can be collected using Multi-Band SWIFT (MB-SWIFT)4 during DBS of the rat brain.Methods
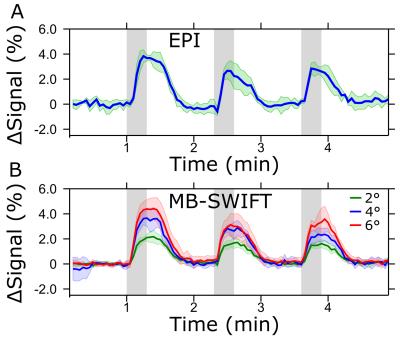
Three-channel tungsten electrodes (diameters 200 µm per channel) were implanted into the rat ventral posteromedial nucleus (vpm; ML 2.8 mm, AP -3.4 mm, DV -5.8; n = 5). The stimulation paradigm consisted of 3 blocks of 60 s of rest and 18 s of stimulation, ending with an additional rest period. The electrode was driven monopolar using 50 µs square pulses repeated at 20 Hz with an amplitude of 0.5 mA/channel. The parameters of MB-SWIFT were: TR = 0.97 ms and 3076 spokes per volume, resulting in temporal resolution of 3 s, bandwidth (BW) = 192 kHz, matrix size = 643 and FOV = 6.4 x 3.5 x 3.5 cm3. Separate trials were made with flip angle = 2°, 4° and 6°. Excitation was performed with a chirp pulse gapped into four 2.6 µs sub-pulses. Two-fold oversampling was used during acquisition in the gaps of 32/BW duration. The post-correlation FID consisted of 32 points. SE-EPI parameters were: TE = 35 ms, TR = 1.5 s, two shots, resulting in temporal resolution of 3 s, BW = 250 kHz, matrix size = 64 x 64, FOV = 3.5 x 3.5 cm2, slice thickness = 1 mm and 11 slices. The resulting data were post-processed in SPM including smoothing with a [2 1 1] pixel FWHM Gaussian kernel, with slice time correction and motion correction for SE-EPI. Because MB-SWIFT is a 3D radial pulse sequence, it is practically motion insensitive, and thus the motion correction was unnecessary. For time series analysis, only activated pixels were used. MB-SWIFT images were reconstructed using gridding and iterative FISTA algorithm5 (3-13 iterations).Results
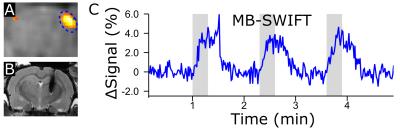
MB-SWIFT exhibited dramatic improvement of the image quality in the presence of an implanted electrode as compared to SE-EPI (Figure 1). The activated areas obtained with MB-SWIFT and SE-EPI were similar. The time series of the activation in the ipsilateral side also exhibited similarity between MB-SWIFT and SE-EPI, although a clear flip angle dependence of the activation amplitude was seen with MB-SWIFT (Figure 2). The functional contrast-to-noise ratio during the first stimulation period for SE-EPI was 10.5 ± 2.4, and for MB-SWIFT 15.8 ± 5.7 (2°), 24.3 ± 16.7 (4°) and 26.5 ± 10.7 (6°). In one rat MB-SWIFT data were acquired with a temporal resolution of 1 s (6°, 1010 spokes, no pre-processing, 13 FISTA iterations) showing excellent agreement with the data obtained using 3 s temporal resolution and no significant deterioration in image quality (Figure 3).Discussion
MB-SWIFT can achieve higher BWs than SWIFT due to much lower RF-pulse duty cycle enabling fMRI in the presence of an electrode. Here, 192 kHz BW was used, and even higher BWs could be achieved within the limitation of gradient heating. High bandwidths and near zero-TE of MB-SWIFT may also aid in post-operational structural imaging for assessment of DBS electrode implantation. MB-SWIFT is a 3D radial sequence and thus high temporal resolution is constrained. However, the lack of contrast in the brain enables relatively high undersampling and thus good temporal resolution can still be achieved. Temporal resolution can be even further improved in combination with compressed sensing. In addition, MB-SWIFT provides relatively high SNR because it acquires data almost constantly throughout the temporal segment, and therefore MB-SWIFT is inherently suitable for fMRI studies. Based on the strong flip angle dependence of MB-SWIFT functional contrast, the main source of the functional contrast is likely due to increased inflow/cerebral blood flow. An experiment eliminating flow effects, such as using a vasodilator, will enable verification of this mechanism.Conclusion
MB-SWIFT shows great potential for fMRI studies in the presence of strong susceptibility variations such as those occurring around implanted leads. In this work we demonstrated that good temporal resolution and robust activation maps can be achieved with MB-SWIFT around implanted electrodes. Further research is needed to confirm that inflow is the major mechanism generating the observed fMRI contrast with MB-SWIFT.Acknowledgements
This work was supported by the following sources: NIH grants: P41-EB015894, P30-NS057091; MICROBRADAM; UEF-Brain Pool; WM KECK Foundation and MnDRIVE post-doctoral fellowship to LJL.References
1. Lai, H. Y., Younce, J. R., Albaugh, D. L. et al. Functional MRI reveals frequency-dependent responses during deep brain stimulation at the subthalamic nucleus or internal globus pallidus. Neuroimage. 2014;8411-18.
2. Kahan, J., Urner, M., Moran, R. et al. Resting state functional MRI in Parkinson's disease: the impact of deep brain stimulation on 'effective' connectivity. Brain. 2014;137(Pt 4): 1130-1144.
3. Mangia, S., Chamberlain, R., De Martino, F. et al. ISMRM Vol. 3187 (Melbourne, 2012).
4. Idiyatullin, D., Corum, C. A. and Garwood, M. Multi-Band-SWIFT. J Magn Reson. 2015;25119-25.
5. Beck, A. and Teboulle, M. Fast gradient-based algorithms for constrained total variation image denoising and deblurring problems. IEEE Trans Image Process. 2009;18(11): 2419-2434.
Figures