0154
Ultra-high resolution blood volume fMRI and BOLD fMRI in humans at 9.4 T: Capabilities and Challenges1SFIM, NIMH, Bethesda, MD, United States, 2MBIC, Maastricht University, Netherlands, 3Scannexus, Maastricht, Netherlands
Synopsis
FMRI at ultra-high field strengths of 9.4 T allows functional imaging with submillimeter spatial resolutions. CBV sensitive VASO-fMRI has been suggested to be weighted towards locally specific microvasculature changes close to neural activity changes. Hence, we sought to combine the high physiological specificity of CBV-fMRI with the high signal-to-noise ratio of 9.4 T imaging. In our experiments, we could identify and discuss numerous technical challenges of CBV-fMRI at 9.4 T regarding constraints of RF fields and VASO contrast generation. With the application of advanced imaging methods, we show promising functional results with clearly visible cortical depth-dependent activity patterns.
Purpose
Functional blood volume imaging with VASO [1] can benefit two-fold from imaging at 9.4 T: A) the supralinear increase in image signal-to-noise ratio (SNR) [2] (approx. 80% higher compared to 7 T [2]) allows higher resolutions in the thermal-noise-dominated regime of submillimeter voxels; B) the longer T1 values and the correspondingly longer-lasting inversion-recovery provides more time for the blood to refill the vasculature.
However,
before applying VASO on a human 9.4 T scanner, numerous technical limitations
must be identified, assessed and accounted for. For instance: 1.) SAR
constraints, 2.) B1+ inhomogeneity, 3.) limited inversion
efficiency, 4.) strong BOLD contaminations, and 5.) faster T2*-decay
during readout. The purpose of this study is to implement, apply, and evaluate
a CBV-sensitive fMRI method in humans at 9.4 T. This is done with the focus to assess and
discuss the feasibility of ultra-high resolution depth-dependent fMRI in light
of the aforementioned 5 challenges.
Methods
Two experiments were performed on a 9.4 T Siemens scanner with a Dual-row 16-channel Tx/31-channel Rx array coil [3]. The aforementioned challenges were addressed as follows: 1.) conservative SAR limits as described in [4] were adhered by exploiting the lower flip angles of a 3D-EPI readout and refraining from the conventional 2D-EPI approach [5]. 2.) B1+ was homogenized with static phase shimming. B0- and B1-mapping were done using a dual-echo GRE and transmit phase-encoded DREAM, respectively, using the workflow and parameters described in [6]. 3.) Inversion efficiency was kept above 85% by customizing an adiabatic TR-FOCI pulse [7] for lower SAR at 9.4 T, including an increased adiabaticity with a reduced bandwidth and phase skip of 30º. 4.) The BOLD effect was assessed and removed from VASO with an interleaved acquisition approach every 1.5 s [8], i.e. only every second volume acquisition was following an inversion pulse 5.) Fast EPI readout was enabled by high-performance head gradients (AC84-mk2, 80 mT/m, 330 mT/m/ms) and GRAPPA-2 with FLASH-based ACS scan [9]. The sequence parameters were: TE=21 ms, in-plane resolution: 0.74x0.74 mm2, TI1/TI2/TR=1.2/2.7/3.0 s assuming blood T1 = 2.3 s [10]. 12 1.7 mm slices were positioned perpendicular to the primary motor cortex. To determine T1, a Bloch-equation model of the sequence was fitted to the multi-TI data [11]. Activity was assessed during a 12 min finger-tapping task (30 s activation vs. 30 s rest). Layer analysis was done in EPI space similar to [12].Results
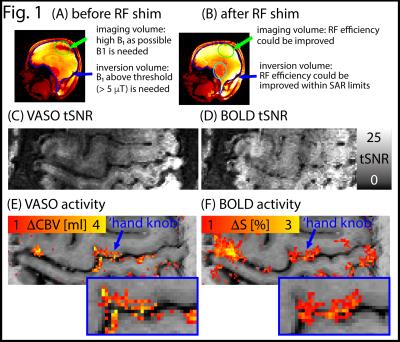
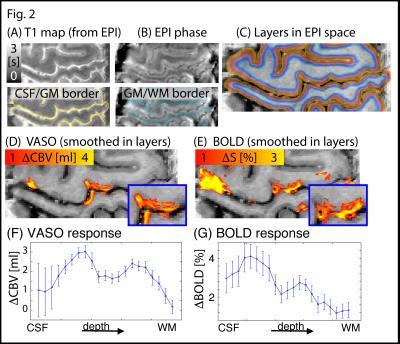
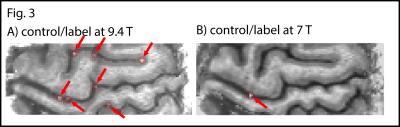
RF shimming improved B1+ homogeneity in the areas of interest (green and blue highlighted regions in Fig. 1A-B). The temporal stability of simultaneous VASO and BOLD images are depicted in Fig. 1C-D. TSNR is high enough to detect tapping induced activity in the motor cortex across the cortical depth (Fig. 1E-F) and layer dependent activity features are visible. T1-maps from the multi-TI functional data in EPI space allows spatially matched layering-analysis (Fig. 2A-C). Spatial smoothing within cortical layers makes the depth-dependent activity modulation clearly visible on a single-slice individual-participant basis (Fig. 2D-E). Corresponding cortical profiles are depicted in Fig. 2F-G. In label-vs.-control images, the functional VASO data showed clear signs of in-flowing not-inverted intravascular blood signal (Fig. 3). While these contaminations are still confined to microvessels outside GM tissue only, they are traveling further downstream compared to 7 T.Discussion
The results shown here suggest that with careful assessment of constraints in RF transmission and CBV-specific contrast generation, VASO fMRI can be successfully applied in humans at 9.4 T. While most of the challenges specific to 9.4 T VASO could be addressed, further improvements can be expected by, for instance, a) use of a dual RF shim for “global” inversion vs. “local” excitation to further increase SAR efficiency, and b) reducing TI1 might further minimize inflow effects.Discussion
We could identify and address the unique challenges for VASO at 9.4 T on human volunteers. High-resolution maps of CBV and BOLD signal changes show promising detectability of cortical depth-dependent response variations. The results shown here suggest, that VASO at 9.4 T will be a useful tool to approach activity mapping in the mesoscopic regime of the human brain.Acknowledgements
Supported by the NIMH Intramural Research Program. Kâmil Uludag receives funding by the Netherlands Organization for Scientific Research NWO: VIDI 452-11-002.Program.References
[1] Lu et al., MRM, 2003, 50:263-274;
[2] Pohmann et al., MRM, 2016, 75:801-809;
[3] Shajan et al., MRM, 2014, 71:870-879;
[4] Hoffmann et al., NMR Biomed, 2015, 29:1131-1144;
[5] Poser et al., NeuroImage, 2010, 51:261-266;
[6] Tse et al., Magn Reson Mater Phy, 2016, 26:333-345;
[7] Hurley et al., MRM, 2010, 63:51-58;
[8] Huber et al., MRM, 2014, 72:137-148;
[9] Ivanov et al., ISMSM, 2015. #2059;
[10] Li et al., MRM, 2016, 76:270-281;
[11] Huber et al., ISMRM, 2016, #633;
[12] Huber et al., NeuroImage, 2015, 107:23-33.
Figures