0146
Probing the BOLD-CVR response to progressive hypercapnia in patients with uni-lateral carotid artery occlusions1Radiology, University Medical Center Utrecht, Utrecht, Netherlands, 2Spinoza Center for Neuroimaging, Amsterdam, Netherlands, 3Radiology, University Medical Center Utrecht, Netherlands, 4University Medical Center Utrecht, Netherlands
Synopsis
We examine the BOLD-CVR response to a progressively increasing vascular stimulus between individuals with carotid artery occlusions and healthy, age/gender-matched controls. Using this paradigm, we aim to understand finer-scale interactions between impaired versus healthy cerebral hemispheres at different vascular stimulus magnitudes.
Introduction
The body of research concerning static and dynamic blood flow properties (measured using BOLD-MRI) in response to vasoactive stimuli is growing. Changes in blood flow characteristics can be linked to disease conditions, thus serving as potential clinical biomarkers. Beyond cerebrovascular reactivity responses in general, of particular interest are response delays stemming from distribution patterns(1), as well as vascular response rates due to impairment(2). A suitable patient model through which to study the effects of disease on vascular impairment are patients with carotid artery occlusions(3). Here, we extend previously published work by examining the regional BOLD-CVR response to a progressively increasing vascular stimulus between individuals with carotid artery occlusions and healthy, age/gender-matched controls. Using this paradigm, we aim to understand finer-scale interactions between impaired versus healthy cerebral hemispheres at different vascular stimulus magnitudes.Methods
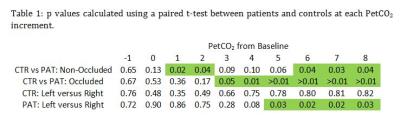
BOLD timeseries data was acquired using a dual-echo PCASL sequence (3T Philips Achieva, 3x3x7mm3, TE/TR: 13.84ms, 36.28ms/4000ms, 11 slices, FA: 90) throughout a targeted breathing challenge (RespirActTTM, Thornhill Research, Toronto) in 7 asymptomatic patients (1 female) with unilateral carotid artery occlusions (66yrs: 52-75) and age/gender matched controls (69ys: 64-76) (4). The breathing protocol consisted of a 120s baseline, a 75s ramp targeted at 10mmHg above resting PetCO2, 100s plateau and then a return to baseline. Multi echo label and control images were averaged(5) to generate BOLD timeseries data which was motion corrected using FSL (MCFLIRT). Subsequent timeseries data was registered to a 2mm MNI-152 atlas. Occluded and healthy hemispheres (and MNI sub-regions) were grouped and BOLD-CVR response curves (6,7) were generated and normalized between subjects by shifting response curves with respect to individual baseline PetCO2 values. Regional data from patients with right sided occlusions were ‘flipped’ such that all occluded hemispheres were considered on the left side of the brain. Significant differences in CVR (p<0.05) at each PetCO2 step from baseline were evaluated using a paired t-test.Results
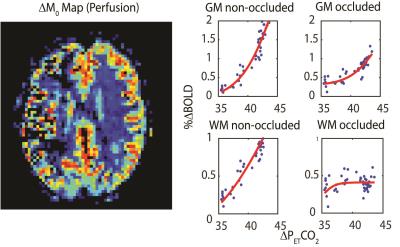
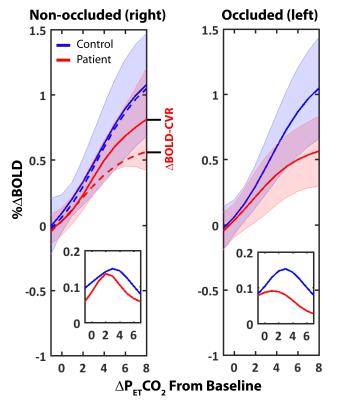
No significant differences in baseline CO2 values (33.4±3.7 and 35.4±1.1 mmHg), baseline O2 values (112±6 and 108±2 mmHg) and whole brain baseline T2* (52±3 and 54±3) were found between controls and patients. A representative BOLD CVR image and the corresponding dynamic BOLD CVR responses of a patient can be found in Figure 1. The amplitude of the progressive CO2 stimulus was 7.5±1.8 and 6.1±1,4 mmHg for controls and patients, respectively (ns). Absolute CVR was higher, and peak CVR (Figure 1, inset) occurred later in controls for both hemispheres. No significant differences were observed for increasing PetCO2 between healthy control hemispheres. A significant difference was observed between the occluded and healthy control hemisphere from 3mmHg above baseline onwards (Figure 2, left panel and Table 1). Ipsilateral versus contralateral responses in patients showed significant differences from 5mmHg above baseline onwards (Figure 2, right panel and Table 1). A mixed effect at increasing PetCO2 was observed in the non-occluded hemisphere between controls and patients (Table 1).Discussion
Our main finding is that significant differences in the BOLD-CVR response to progressive hypercapnia were present in cerebral hemispheres located ipsilateral to carotid artery occlusions. Furthermore, non-occluded hemispheres of patients also showed reduced CVR. Our findings support those reported by Sam et al(3) who showed that single sided occlusions have a global effect on CVR, however, provide additional information regarding the stimulus level at which impairments become evident. The use of a slowly increasing vascular stimulus has several benefits for evaluating CVR; for example, patients with occlusions are affected by arterial transit delays which might confound ASL based CVR measurements. Furthermore, the use of abrupt CO2 increases, such as those associated with block hypercapnic stimuli, can lead to a mixture of dynamic and static blood flow effects. Longitudinal studies investigating the relationship between contralateral impairment and risk of ischemic event may shed light onto compensatory mechanisms relating to blood flow redistribution. Furthermore, deeper investigation is required to determine whether healthy territories, or even those downstream of occlusion, suffer true vascular impairments relating to disease or are merely in a chronic, pre-dilated state.Acknowledgements
No acknowledgement found.References
1. Mazerolle, EL et al., Impact of abnormal cerebrovascular reactivity on BOLD fMRI: a preliminary investigation of moyamoya disease. Clinical physiology and functional imaging 2016.
2. Duffin J, et al., The dynamics of cerebrovascular reactivity shown with transfer function analysis. NeuroImage 2015;114:207-216.
3. Sam K, et al., Reduced contralateral cerebrovascular reserve in patients with unilateral steno-occlusive disease. Cerebrovascular diseases (Basel, Switzerland) 2014;38(2):94-100.
4. De Vis JB, et al., Calibrated MRI to evaluate cerebral hemodynamics in patients with an internal carotid artery occlusion. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2015;35(6):1015-1023.
5. Bhogal AA, et al., The BOLD cerebrovascular reactivity response to progressive hypercapnia in young and elderly. NeuroImage 2016;139:94-102.
6. Bhogal AA, et al., Investigating the non-linearity of the BOLD cerebrovascular reactivity response to targeted hypo/hypercapnia at 7T. NeuroImage 2014;98:296-305.
7. Sobczyk O, et al., A conceptual model for CO(2)-induced redistribution of cerebral blood flow with experimental confirmation using BOLD MRI. NeuroImage 2014;92:56-68.
Figures